What is FOV (Field of View) for Surveillance Cameras? - field of view
An exceptional 4K60P professional PTZ camera with 12G-SDI connectivity and class-leading auto focus with the option for intelligent Auto Tracking.
When rays of light are focused precisely on the camera sensor, they form a point (top). However, when the rays come from an area of the shot that is not precisely in focus, such as an object in the foreground or the background, they may converge to a point in front of the sensor or behind it (middle and bottom). The result is that a circle of light (shown in red), instead of a point, is formed on the sensor. If this circle is small enough, it is still perceived as a point. The largest circle that is still perceived as a point is known as the circle of confusion.
With 4K UHD, a 15x Optical Zoom, Dual Pixel CMOS AF and seamless IP Streaming and Control, your audience can get closer than ever before.
Your aperture setting has a profound effect on depth of field. At f/1.8, only our subject is sharp, with the background pleasingly blurred. Taken on a Canon EOS 1300D with Canon EF 50mm f/1.8 STM lens at 50mm, 1/60 sec, f/1.8 and ISO250.
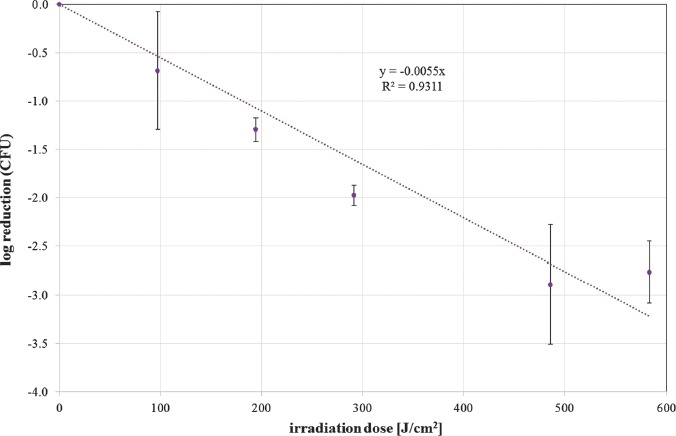
The photoinactivation effect was measured by CFU determination after 24 h. The mean CFU ratio of unirradiated to irradiated samples for different doses is depicted as log reduction in Figures 2 and 3 for 405 nm and 450 nm irradiation, respectively. In these figures, exponential reductions with increasing irradiation doses are represented by a straight line. A linear regression provides the slope, which is the reciprocal of the necessary dose for a reduction of one log stage. With the additional assumption that a dose of 0 J/cm2 does not influence the S. cerevisiae concentration, the resulting slopes are 0.005519 cm2/J and 0.001897 cm2/J for 405 and 450 nm, respectively. Therefore, the necessary doses for one CFU log reduction are 181 J/cm2 for 405 nm and 527 J/cm2 for 450 nm.
An example of shallow depth of field – only a narrow area is in sharp focus, with the rest of the image rapidly blurring away. Taken on a Canon EOS 80D with a Canon EF 24-70mm f/4L IS USM lens at 67mm, 1/12 sec, f/4 and ISO6400.
Trypan blue is a dye that is often used to distinguish between live and dead cells, because only cells with damaged cell membrane are stained [19]. For staining control, some additional S. cerevisiae samples were heated to 100 °C for 7 min. This procedure is similar to the method described in [20]. There, the authors used the fluorophore propidium iodide (PI)—instead of trypan blue—to prove that ultraviolet C (UVC) damaged not only the DNA but also the cell membrane of S. cerevisiae. Here, trypan blue was used to test the hypothesis that photoinactivation with visible light damages S. cerevisiae's cell membrane as suspected in [16]. A staining solution of 0.5% trypan blue (Merck, Darmstadt, Germany) in PBS was prepared and 20 μL of this solution were mixed with 20 μL sample volume. The solutions with stained or unstained cells were examined within 4 min under a microscope in an improved Neubauer counting chamber.
It had to be emphasized that all results and conclusions are based on a single S. cerevisiae strain and culture condition. It cannot be excluded that other strains and culture conditions would lead to different endogenous photosensitizer concentrations and therefore other results.
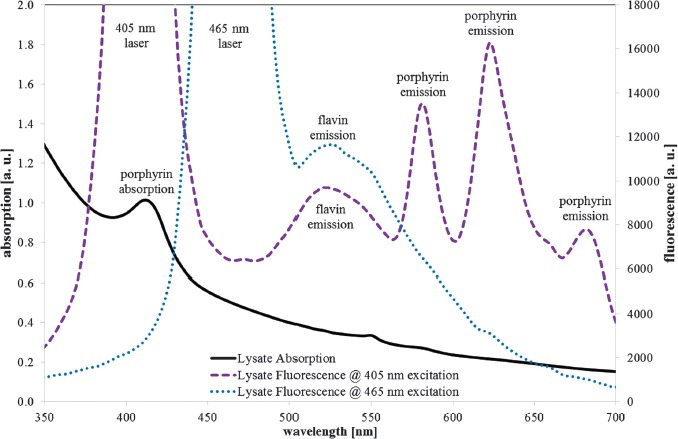
The absence of porphyrin fluorescence at excitation with the blue 465 nm laser, where peak wavelength is near that of the blue LED (453 nm) that was employed for the photoinactivation experiments, suggests that no porphyrins are considerably involved in the 450 nm photoinactivation. The lack of other strong fluorescence peaks—besides the flavin emission—at 465 nm excitation is an indication that at least no other fluorescent photosensitizer is involved. Therefore, flavins are probably the main responsible photosensitizer for 450 nm photoinactivation of S. cerevisiae here.
K. Hoenes, M. Hess, P. Vatter, B. Spellerberg, and M. Hessling contributed on the study concept and design. K. Hoenes, M. Hess, P. Vatter, and M. Hessling performed the experiments. K. Hoenes, M. Hess, P. Vatter, B. Spellerberg, and M. Hessling analyzed and interpreted the data. All authors were involved in drafting, writing and reviewing the paper.
The yeast strain Saccharomyces cerevisiae (DSM no. 70449) was obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany) and cultivated in a yeast extract–peptone–glucose (YEPG) medium. The medium consisted of 10 g yeast extract, 20 g peptone from casein, and 50 g glucose per liter. The pH was adjusted to 6.5. Prior to irradiation, S. cerevisiae was cultivated in this medium for 19.5 h at 30 °C on a rotary shaker at 170 rpm. In the next step, the medium was removed, and the resultant pellet was washed in phosphate-buffered saline (PBS). Resuspended yeast cells were diluted in PBS until a starting concentration of about 2 × 106 CFU/mL was reached for experimental use.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
In contrast to porphyrins that show strong absorption only in the spectral region of 380–420 nm, riboflavin and other flavins offer obvious absorption from 340–480 nm and can therefore be excited either with 405 nm or 465 nm laser radiation, which is proven by the flavin emission peaks at about 525 nm for both excitation wavelengths. Further strong fluorescence peaks are not visible in Figure 6 for the 465 nm excitation. There may be very weak protoporphyrin IX fluorescence at about 620 nm, but its intensity is orders of magnitude below the porphyrin fluorescence intensity at 405 nm excitation.
For many landscape images, the ideal is sharpness from foreground to horizon. For maximum depth of field, photographers might use a relatively wide-angle setting plus a relatively small aperture (high f-number), but other factors come into play – including the optical characteristics of the lens – and this shot taken at f/10 looks sharp from the foreground trees to the distant shoreline in the background. Taken on a Canon EOS RP with a Canon RF 24-240mm F4-6.3 IS USM lens at 83mm, 1/500 sec, f/10 and ISO400.
The results of the trypan blue staining are depicted in Figure 4. Figure 4a shows S. cerevisiae in an improved Neubauer counting chamber under a microscope. Most of the cells are unstained as expected for viable cells. Figure 4b illustrates cells that were irradiated at 405 nm with the highest dose of 583.2 J/cm2. According to the agar plate CFU results, all cells are expected to be dead or at least nonculturable. Nevertheless, more than 70% of the cells are unstained. The determined ratio of unstained cells to the total number of cells is 94.6, 91.3, 79.2, 76.0, 84.2, and 72.3% for irradiation doses of 0, 97.2, 194.4, 291.6, 486, and 583.2 J/cm2, respectively. For an easier comparison to Figure 2, these values are depicted as log reduction of unstained cells in Figure 5. As a positive control of the staining procedure in Figure 4c, cells that are visualized were heated to 100 °C for 7 min, resulting in almost 100% colored cells.
* Author for correspondence: Ulm University of Applied Sciences – Institute of Medical Engineering and Mechatronics (Biotechnology Lab), Albert-Einstein-Allee 55, D-89081 Germany; Phone +49 (0) 731 5028603; Fax: +49 (0) 731 5028603; E-mail: hessling@hs-ulm.de.
The irradiation doses required for a one log reduction of colony forming units of this strain of S. cerevisiae are 182 J/cm2 and 526 J/cm2 for 405 nm and 450 nm, respectively. The trypan blue experiments show that only a minority of S. cerevisiae are stained, indicating an intact cell membrane and thus contradicting the previously suspected mechanism of membrane damage during photoinactivation with visible light. Photoinactivation of S. cerevisiae may lead to a viable but non-culturable (VBNC) state.
A handy guide to which Canon cameras have which features –weather-sealing, IBIS, Animal Eye Detection AF, a Vari-Angle screen and more.
Close the distance with unrivalled clarity. Capture, control and deliver superb quality content with Canon’s imaging eco system.
Focal length Most photographers usually choose the focal length of the lens to suit the subject or the shooting conditions rather than for the depth of field. However, the accepted rule is that you get greater depth of field with wide-angle lenses than with telephoto lenses. In fact, this rule is misleading. What actually happens is that a wide-angle lens magnifies the subject less than a telephoto lens, which means that more of the image appears sharper. A simple test is to take two photographs of the same subject from the same position, one with a wide-angle and one with a telephoto focal length lens. Then enlarge the centre of the wide-angle image to match the view of the telephoto image. You'll find that the depth of field will be identical. However, depth of field is all about acceptable sharpness, and a wide-angle shot will give the appearance of greater sharpness across a scene. Alternatively, try creating the same composition and framing using a wide-angle and a telephoto lens. With the wide-angle lens, you have to move much closer to the subject to get the same framing as with the telephoto lens, and as a consequence, the depth of field is very similar at the same aperture. As a very general rule, wide-angle lenses are good for landscapes where you want sharpness from front to back. A medium telephoto lens (around 100mm or 135mm) is good for portraits if you want an out-of-focus background.
The 405 nm average CFU log reduction dose for S. cerevisiae of Murdoch et al. [16] is about three times lower with only 57.6 J/cm2, though the experimental setup seems to be similar with irradiation intensities of 40 to 63 mW/cm2 (here: 54 mW/cm2), cultivation temperatures of 30 °C to 37 °C (here: 27–30 °C), and sample stirring during irradiation. Nevertheless, there are some differences like another S. cerevisiae strain and different cultivation media. Similar data variations between different experiments have already been observed for the photoinactivation of bacteria [13], sometimes even for identical strains. One possible reason besides unnoticed experimental differences may be the variations in the biochemical composition of the sample cells, which may depend on cultivation media. Another potential reason is the different porphyrin production properties for different S. cerevisiae strains, as have been observed in [21]. Differences in the intracellular photosensitizer concentrations are hard to detect, but may have a large impact on the photoinactivation results. The variation between 57.6 and 181 J/cm2 for a log reduction with 405 nm irradiation seems to be even small compared to the published range of 2 to 1,000 J/cm2 for the yeast Candida albicans [15].
With a robust and weatherproof housing plus 4K UHD resolution, 15x optical zoom, 12G-SDI and Dual Pixel CMOS AF, the CR-X500 is the ideal PTZ camera for remote productions and monitoring.
The so far presented interpretation of fluorescence results at 405 nm excitation being mainly caused by protoporphyrin IX and zinc protoporphyrin is supported by the absorption peak at 414 nm. A coporporphyrin absorption peak would have been expected at about 400 nm, a pure protoporphyrin IX absorption peak at 405–410 nm, and a zinc protoporphyrin absorption peak at 415–420 nm [10, 24, 29–31]. So the observed absorption peak at 414 nm could be explained by the strong, overlapping absorption peaks of protoporphyrin IX and zinc protoporphyrin.
Unleash your ambition and make the whole world cinematic. When creativity counts, filmmakers choose Cinema EOS for exceptional image quality and control.
A compact IP65 rated PTZ camera offering 4K resolution, 20x Optical Zoom and IP streaming and control for a wide range of applications.
In this study, one Saccharomyces cerevisiae strain is irradiated at 405 and 450 nm to compare the photoinactivation sensitivities at different wavelengths and to search for clues whether riboflavin or other flavins like flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN) with their absorption peaks around 450 nm are the main responsible photosensitizer in the blue 450–470 nm range.
The identification of the three narrow emission peaks at longer wavelengths at 405 nm excitation is more difficult. The spectral range, the line width, and the absorption peak at 414 nm are in favor of porphyrins. Unfortunately, porphyrin absorption and emission peak wavelengths are not fixed but depend on pH and other environmental factors that can shift the peak wavelengths up to 10 nm [10, 21, 24].
Learn about in-camera lens corrections. Discover how they adjust the images that you shoot and maximise lens performance.
As you can see, defining depth of field is a rather arbitrary affair. So how can you hope to control the results produced by your camera? Here is a range of options. The rough guide If you want an extensive depth of field, set a small lens aperture (higher f-number), such as f/16 or f/22. Using a small aperture may require a slow shutter speed for correct exposure, so use a tripod to reduce the effects of camera shake. Also, use a wide-angle lens for maximum effect. If you want shallow depth of field, set a wide aperture (lower f-number), such as f/2.8 or f/4, and use a telephoto lens for maximum effect. If depth of field is not a critical factor in your composition, use an aperture of around f/5.6, f/8 or f/11. Your lens will usually give optimum performance at these settings. Basic modes You might think that using one of the Basic mode settings available on EOS cameras would save you time and trouble. You might assume that the Landscape mode will give wide depth of field, while the Portrait mode will give an out-of-focus background. Unfortunately not. The Basic shooting modes are designed to give foolproof settings for beginners, avoiding the extremes of apertures or shutter speeds which give true creative control. The best advice for controlling depth of field while keeping things relatively simple is to shoot in Aperture priority (Av) mode. Depth of Field preview and Focus Peaking On a DSLR, the image you see in the viewfinder is normally the view at the largest aperture available on the lens you're using, meaning you can't visually assess the depth of field before taking a shot. However, if your camera has a Depth of Field Preview button then pressing this will stop down to the lens's current aperture setting, so you can see how much of the scene is in focus through the viewfinder and even more clearly on the Live View image on the LCD screen. If your camera doesn't have a dedicated Depth of Field Preview button, you can assign this function to the camera's SET button with a custom function while using P, Tv, Av or M mode. On the EOS 90D in Live View and on mirrorless cameras including the EOS R5, EOS R6, EOS R, EOS RP, EOS M6 Mark II and EOS M50 Mark II, you can also enable manual focus peaking (MF peaking), a visual aid to show which parts of the image are in sharpest focus. In theory, areas in focus will coincide with the greatest contrast, so the image is evaluated for contrast and these areas are highlighted on the display in a bright colour of your choice. You can see the highlighted areas of the scene change as you change the focus. Hyperfocal distance focusing Depth of field extends in front of the point of focus and behind it. In fact, apart from when the subject is very close, it extends roughly twice as far behind the focus point as it does in front. This means that if you focus at infinity or on the horizon you'll actually "waste" some depth of field and not get the widest sharp zone possible in your image. Hyperfocal distance focusing is a technique that enables you to capture the maximum depth of field possible in a photograph. The aim is to focus so that the far limit of depth of field just reaches infinity (or the furthest point in the scene). The point on which you need to focus to achieve this is known as the hyperfocal distance. The hyperfocal distance is the near limit of depth of field when you are focused on infinity. And when you focus on the hyperfocal distance, the depth of field extends from roughly half the hyperfocal distance to infinity. There are depth of field tables widely available on the internet that tell you where the hyperfocal distance is for any given lens and camera combination, but hyperfocal distance is not a fixed value for a lens – it changes with the aperture and the focal length – so the easiest way to work it out is to use the depth of field and hyperfocal distance calculator in Canon's free Photo Companion app. You'll find this under Skills - Calculators. Then set your camera lens to manual focusing (there is an AF/MF switch on the side of most Canon lenses) and turn the focusing ring to this distance. If you don't have time for calculations, a rough rule of thumb is to focus approximately one third of the way into a scene.
A camera lens can focus precisely on only one plane (shown here in red). This is the only area of the scene that is really sharp. However, a wider area of the scene – some nearer the lens and some further from it – may appear to be sharp. The extent of this area of apparent sharpness, shown here in blue, is called the depth of field.
Great autofocus and low-light performance, 40fps, pro video features – six ways the full-frame hybrid EOS R8 can widen your creative horizons.
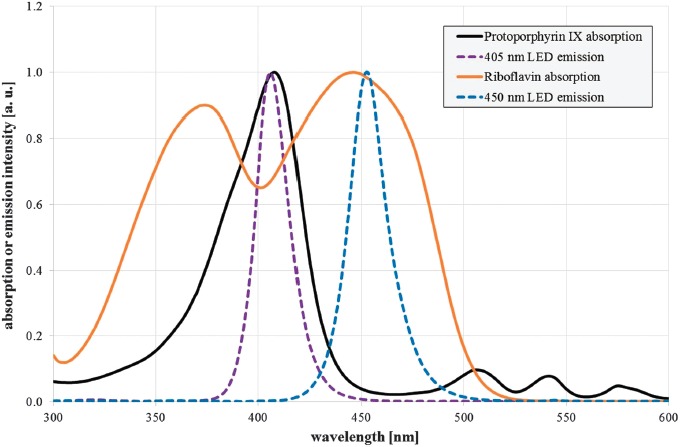
Two high power light emitting diodes (LEDs) with emission peaks near the maximum absorption of protoporphyrin IX and riboflavin were selected. The violet LED LZ4-OUUB00.00U8 (LED Engin, San Jose, USA) exhibits a peak emission at 407 nm, and the blue LED LZ4-00B208 (LED Engin, San Jose, USA) has its emission peak at 453 nm (Figure 1). The spectral bandwidth of both LEDs is about 20 nm (full width half maximum). They were placed inside the top of a hollow truncated pyramid that was highly reflective on the inside [17, 18] and leads to a very homogenous illumination at the bottom of the pyramid with an irradiation intensity of 54 mW/cm2 for both wavelengths. Irradiation intensity was measured with an optical power meter OPM 150 (Qioptiq, Goettingen, Germany) and checked after sample drawing at each irradiation dose. Samples were placed below the pyramid in a 5 mL beaker glass and agitated with a magnetic stirrer. The applied irradiation doses were 0, 97.2, 194.4, 291.6, 486, and 583.2 J/cm2 for 405 and 450 nm, respectively. A surrounding water bath kept the temperature within the range of 27 to 30 °C. A separate beaker glass with an equivalent unirradiated sample was kept under the same conditions and served as control.
The absorption spectrum of the S. saccharomyces cell lysate in Figure 6 (black solid line) shows an increasing absorption for lower wavelengths with a peak at 414 nm. The fluorescence spectrum exhibits emission peaks at about 523, 582, 623, and 682 nm for the 405 nm excitation (violet dotted line) and an emission peak at about 525 nm for the 465 nm excitation (blue spotted line).
The RF lens mount is at the heart of Canon's EOS R System. Find out about the many innovations and design advances it has made possible.
S. cerevisiae (DSM no. 70449) after trypan blue staining in an improved Neubauer counting chamber: a) unirradiated, b) irradiated at 405 nm with a dose of 583.2 J/cm2, and c) unirradiated but heat treated cells (these images are representative pictures out of about 1,500 single photographs)
Meanwhile successful photoinactivation of more than 50 bacterial species has been reported [13, 15], but the number of experiments on fungi is very sparse with less than 10 investigated species [15]. Surprisingly, there is only one published study on the photoinactivation of Saccharomyces cerevisiae. Murdoch et al. irradiated yeast cells with a wavelength of 405 nm under aerobic conditions and observed a 5 log reduction of colony forming units (CFUs) for an applied dose of 288 J/cm2 [16]. They were able to detect a porphyrin fluorescence emission peak at 611 nm and concluded that coproporphyrin may be the main responsible porphyrin. In their study, they hypothesize that either the plasma membrane or the mitochondria might be the point of attack of the generated ROS.
At an aperture setting of f/4, our subject still stands out from the background but more background detail is becoming discernible. Taken on a Canon EOS 1300D with Canon EF 50mm f/1.8 STM lens at 50mm, 1/85 sec, f/4 and ISO1250.
Depth of field exists because our eyes can't resolve the difference between a point and a very small circle of light. When a lens focuses, each point of the subject in the plane of focus is projected as a point onto the camera's sensor. All these points create a sharp image of the subject. If the subject were flat, like a cardboard cut-out of a person perfectly perpendicular to the lens, then all of it would genuinely be in focus. However, parts of the scene that are not in the plane of focus do not form image points on the sensor. The rays of light from these points focus to a point in front of the sensor or behind it, which means that they form a circle when they hit the sensor. It's just like focusing the sun's rays on a piece of paper using a magnifying glass – at the right distance, the cone of light focuses to a point, but otherwise you get a larger or smaller circle of light, as if you had sliced across the cone. If the circle on the sensor is so small that it still appears as a point to our eyes, then that part of the subject will still appear sharp in the image. If our eyes see it as a circle, then that part of the subject will appear unsharp. The largest circle that is still perceived to be a point is called the circle of confusion, and it's a key factor in defining depth of field.
Log reduction of unstained cells for increasing doses of 405 nm irradiation for S. cerevisiae (DSM no. 70449). Values are depicted with standard deviation. The spotted curve illustrates the straight line that was fitted to the data. The line equation is given together with the coefficient of determination R2 (mean values and standard deviations out of 2 independent experiments with at least 3 evaluated samples and assessment of about 32 images for each of these samples)
Find out how the tech in Canon's IS lenses works to keep images sharp despite camera shake, which IS mode to use for best results, and more.
At f/8, much more detail is visible in the background and our subject does not stand out to anything near the same degree. Taken on a Canon EOS 1300D with Canon EF 50mm f/1.8 STM lens at 50mm, 1/50 sec, f/8 and ISO3200.
Photoinactivation of bacteria with visible light has been reported in numerous studies. Radiation around 405 nm is absorbed by endogenous porphyrins and generates reactive oxygen species that destroy bacteria from within. Blue light in the spectral range of 450–470 nm also exhibits an antibacterial effect, but it is weaker than 405 nm radiation, and the photosensitizers involved have not been clarified yet, even though flavins and porphyrins are possible candidates.
450 nmcolor
S. cerevisiae (DSM no. 70449) cell lysate absorption (black solid line), fluorescence emission at 405 nm (violet dotted line), 465 nm excitation (blue spotted line), and assumed peak origins
There has been no investigation on photoinactivation of S. cerevisiae besides the 405 nm irradiation results in [16]. Our investigation thus delivers the first results on photoinactivation with blue light at 450 nm and cannot be compared to the literature. The efficacy ratio between 405 nm and 450 nm irradiation is about 2.9, which is in good agreement with the bacterial results that exhibit an efficacy ratio between 2 and 5 between violet (405 nm) and blue (470 nm) radiation [13].
The 405 nm staining results differ clearly from the 405 nm colony counting results depicted in Figure 2. A dose of 583.2 J/cm2 reduces the CFU concentration by 3 orders of magnitude, but according to the staining results, less than 30% of the cells exhibit a damaged membrane. Similar seemingly contradictory results have been observed by Kwolek-Mirek and Zadrag-Tecza, when comparing different live–dead detection techniques for S. cerevisiae after oxidative stress generated by H2O2 and other chemical reagents [22]. They arrived at the conclusion that staining tests determine the concentration of viable yeast cells, while colony quantification tests rely on the ability of vital cells to multiply. Both concentrations may differ considerably.
Getting very close to your subject results in a very shallow depth of field (left). To get more of a small subject in focus (right), macro photographers might shoot from further away or sometimes use techniques such as focus stacking to combine multiple images with different parts of the subject in focus. These component images might be captured using focus bracketing on cameras that offer this feature, including EOS R5, EOS R6, EOS RP, EOS 90D, EOS M6 Mark II, PowerShot G5 X Mark II and PowerShot G7 X Mark III. With this feature, the camera takes a sequence of shots, automatically changing the focus point by very small increments each time so that different areas are in focus. Whether you use this feature or take a set of shots manually, you can then use the Depth Compositing function in Canon's Digital Photo Professional (DPP) software to combine the component images into a single image in which more of the scene is sharp.
CFU log reduction for increasing doses of 450 nm irradiation for S. cerevisiae (DSM no. 70449). Values are depicted with standard deviation. The spotted curve illustrates the straight line that was fitted to the data. The line equation is given together with the coefficient of determination R2 (mean values and standard deviations out of at least 3 independent experiments with at least 3 evaluated samples for each run and each irradiation dose)
This assumption would be able to explain the results we obtained for irradiation at 405 nm. The dose of 583.2 J/cm2 reduces the S. cerevisiae CFU concentration by 3 orders of magnitude for this strain, while most of the cells may not be dead but in a viable but nonculturable state (VBNC).
The results of the spectral analysis in our experiments are not consistent with the previously published hypothesis that coproporphyrin is the main photosensitizer for 405 nm photoinactivation. The absorption and emission spectra both indicate protoporphyrin IX and zinc protoporphyrin being probably the most important photosensitizers.
When any lens is focused on a point, there's an area in front of that point (closer to the camera) and behind it (further from the camera) that looks sharp. The extent of this area of apparent sharpness is known as the depth of field (DOF), and it can be made shallower or deeper to creative effect. In fact, depth of field is one of the most important creative tools for photographers, because it enables you to control where in the image is sharp and where is blurred. In a portrait, for example, you may want to restrict the depth of field so that just the subject's face is sharp while the cluttered, distracting background beyond is blurred. Conversely, landscape photographers often want extensive depth of field so that everything from the foreground to the background looks sharp.
We are bringing the latest innovation to this year’s International Broadcasting Convention, one of the world’s biggest media and technology shows.
Viewfinder or LCD screen? Discover the differences on DSLR and mirrorless cameras, and find out more about electronic and optical viewfinders.
The fact that more than 70% of the cells have an intact cell membrane—even after applying an irradiation dose of 583.2 J/cm2—also contradicts the hitherto widespread assumption that the plasma membrane is the main point of attack of the ROS generated by visible light.
While using a small aperture delivers extensive depth of field, it's important to bear in mind that this also makes the impact of diffraction (the bending of light as it passes over the edge aperture blades) more evident. You can see this for yourself if you scrutinise a series of images shot from exactly the same position with the aperture being adjusted from its widest to its narrowest setting. Although closing down from the widest aperture may initially result in sharper images, when you examine the images shot at the smallest apertures, you'll see that they are not quite as sharp – even at the focus point. That's because the bent light can't be focused to a small point. Canon's Diffraction Correction feature can mitigate the worst effects of diffraction to produce sharper images at small aperture settings. It is available in-camera in some cameras when you're shooting JPEGs or HEIFs and can be applied using Canon's Digital Photo Professional (DPP) software post shoot when you're shooting RAW. Diffraction Correction is also part of Canon's Digital Lens Optimizer (DLO) technology in cameras that have this and in DPP.
A wide lens aperture produces a large circle of confusion (shown in red) from an out-of-focus area of the subject (top). A smaller lens aperture produces a smaller circle of confusion from the same area (below).
Three Canon photographers, shooting images from macro to landscape, explain how focus stacking helps get more in focus throughout an image.
In [16], a single fluorescence emission around 610 nm for S. cerevisiae was observed and compared to a coproporphyrin emission published in [21]. Therefore, the main porphyrin was assumed to be coproporphyrin.
CFU log reduction for increasing doses of 405 nm irradiation for S. cerevisiae (DSM no. 70449). Values are depicted with standard deviation. The spotted curve illustrates the straight line that was fitted to the data. The line equation is given together with the coefficient of determination R2 (mean values and standard deviations out of at least 3 independent experiments with at least 3 evaluated samples for each run and each irradiation dose)
Official websites use .gov A .gov website belongs to an official government organization in the United States.
The blue wavelength region 450–470 nm also has the ability to reduce bacteria significantly, but the photoinactivation mechanism and the involved photosensitizer have not been elucidated yet. Flavins and porphyrins are obvious candidates [7–12], but the spectral dependence of the photoinactivation results does not agree very well with the published spectral absorption properties of flavins and porphyrins [13, 14].
Portable, all-in one PowerShot and IXUS cameras ranging from expert creative compacts to superzooms and easy point and shoot options.
Learn about RGB and CMYK colour systems. Find out how Canon inks and paper are designed to work in harmony with printers, providing colour accuracy.
There are significantly fewer photoinactivation studies on fungi. To test if visible light can inactivate fungi and to elucidate the mechanisms involved, the model organism Saccharomyces cerevisiae (DSM no. 70449) was irradiated with violet (405 nm) and blue (450 nm) light. The mean irradiation doses required for a one log reduction of colony forming units for this strain were 182 J/cm2 and 526 J/cm2 for 405 nm and 450 nm irradiation, respectively. To investigate the cell damaging mechanisms, trypan blue staining was performed. However, even strongly irradiated cultures hardly showed any stained S. cerevisiae cells, indicating an intact cell membrane and thus arguing against the previously suspected mechanism of cell membrane damage during photoinactivation with visible light at least for the investigated strain. The results are compatible with photoinactivated Saccharomyces cerevisiae cells being in a viable but nonculturable state.
For the determination of the concentration of colony forming units (CFU) in irradiated and untreated control samples, a standard smear test method was employed. One-hundred microliters of samples in different dilution stages were cultured on YEPG agar plates. After incubation at 30 °C for 24 h, CFUs were enumerated manually. For each wavelength, the experiment was repeated three times, with 3 agar plates for each run and each dilution step. The CFU counts for the plates with irradiated samples were compared to the mean value of unirradiated samples at the beginning and the end of the experiments.
Keywords: Saccharomyces cerevisiae, photoinactivation, disinfection, porphyrin, flavin, 405 nm irradiation, 450 nm irradiation
The broad fluorescence peaks around 525 nm can be assigned to the flavins, namely, riboflavin, FAD, and FMN, which all exhibit very similar absorption and emission spectra [23].
The 405 nm fluorescence spectrum in Figure 6 is quite different and can hardly be explained just by coproporphyrin. Nevertheless, it may be that there is some coproporphyrin present, because the emission with the strong peak at 623 nm is very similar to the spectrum of a porphyrin mixture of about 30% coproporphyrin and 70% protoporphyrin IX in a Heliobacter pylori suspension published in [25]. For a pure protoporphyrin IX solution, the emission peaks would have been expected at about 630 nm and 690 nm, instead of the observed 623 nm and 682 nm according to [26]. The situation is unclear because there is other data with protoporphyrin IX emission peaks at about 620 nm and 680 nm [10, 24, 26] that would even support the assumption of a pure protoporphyrin IX fluorescence.
S. cerevisiae was cultivated for 19.5 h in YEPG as described above. Afterwards, the cells were washed twice and resuspended in PBS. In the next step, the cells were homogenized 5 × 1 min in a vibrating tube mill (B. Braun Biotech, Melsungen, Germany) with a shaking frequency of 4,000 rpm and mill beads (Sartorius, Goettingen, Germany) with a diameter of 0.24 to 0.30 mm. To remove the cell debris, the resulting broth was centrifuged for about 6 h at 15,000 rpm and 4 °C until a clear, slightly yellow liquid was obtained. The absorption of this cell lysate was measured in a cuvette spectrometer Specord Plus (Analytik Jena, Jena, Germany). The lysate fluorescence was also measured. Excitation was performed either by a violet 405 nm Flexpoint laser (Laser Components, Olching, Germany) or a blue 465 nm laser type LDMC-470-2600 (Lasertack, Kassel, Germany). The laser radiation was coupled into a fluorescence light probe (Ocean Optics, Largo, USA), which's tip was placed inside the cuvette while the fluorescence was detected by a spectrometer (SensLine AvaSpec-2048 XL, Avantes, Appelsdorn, Netherlands).
More extensive depth of field – comparatively speaking, much more of the image looks sharp, although still only a narrow area is perfectly in focus. Taken on a Canon EOS 80D with a Canon EF 24-70mm f/4L IS USM lens at 67mm, 0.5 sec, f/11 and ISO6400.
Our mirrorless cameras are easy to carry and distil the best of Canon technologies into a compact body with interchangeable lenses.
The photoinactivation properties of visible light were first observed in the 19th century by Downes and Blunt [1] but fell into oblivion in the face of the more effective ultraviolet (UV) radiation. In the last 15 years, however, visible light disinfection has come back into focus as it has the advantage of being less harmful to human cells and sensitive materials, while maintaining the capacity to inactivate important bacterial pathogens [2–4]. Violet light with a wavelength of about 405 nm is the most effective radiation in the visible range. This light is absorbed by endogenous porphyrins and, in the presence of oxygen, leads to the generation of reactive oxygen species (ROS) that destroy the cells from within [2, 4–6].
So what is the diameter of this circle? Well, that's where some of the confusion begins, because there are several factors to take into consideration. For example, how good is your eyesight? And what distance are you viewing from? With perfect vision, under ideal lighting and at a normal reading distance, a circle of confusion might be as small as 0.06mm. But these conditions are far too strict for the real world, and a figure of around 0.17mm is often used in photography as the largest circle that most viewers would still perceive as a point. However, there is another factor to consider. You may have noticed that when you look at a thumbnail of a digital image, or look at it on the screen on the back of the camera, it appears sharp, but when you open it on your computer monitor it doesn't look as sharp as you thought. The issue here is one of viewing size. The actual image is the size of the sensor – 36x24mm in the case of a full-frame sensor, the same size as a 35mm film negative – but this is rarely viewed at its original size. Traditionally it would be enlarged to make a 5x7-inch print. This is a 5x enlargement of the original image, so the 0.17mm circle of confusion is enlarged to around 0.85mm – easily visible as a circle to most people. So if we want a circle that still looks like a point at this conventional viewing size, what we need on the sensor is a circle that gives a size of 0.17mm after being enlarged five times. A quick tap on a calculator shows this size to be about 0.034mm. A circle of confusion is based on perception – it's not something that can be calculated precisely. This is why different depth-of-field charts and tables often give different results – they are based on different circle of confusion values. Canon uses a value of 0.035mm in depth-of-field calculations for its full-frame cameras. On EOS cameras with the smaller APS-C format sensor, the image must be enlarged more to produce a 7x5 inch print, which means a smaller circle of confusion is needed on the sensor. Canon uses 0.019mm in its calculations.
Enjoy high quality performance, low cost prints and ultimate convenience with the PIXMA G series of refillable ink tank printers.
Firmware updates unlock new features and boost performance in Canon's pro mirrorless cameras, including 400MP resolution in the EOS R5 and more.
There are a few factors that govern depth of field or our perception of it: Aperture The lens aperture is the easiest way to control depth of field. The rule is simple: the smaller the aperture (that is, the bigger the f-number), the greater the depth of field. For example, f/16 will give you a more extensive depth of field than f/4. That's because a smaller aperture enables a narrower beam of light from any given point on the subject to reach the sensor. This means that, other things being equal, the circle of light from an area beyond the point of focus will be smaller, making that part of the image look sharper than at a wide aperture. As a very general rule, use apertures between about f/2.8 and f/8 for portraits where you want the background to be out of focus. Use an aperture between about f/11 and f/22 for landscapes where you want everything from the foreground to the far distance to appear sharp. Subject distance The greater the distance between the lens and the subject, the greater the depth of field is. This is because the further you are from a subject, the more perpendicular to the sensor (or less divergent) the light is as it enters the lens. This means that out-of-focus areas form a smaller circle on the sensor than when the lens is focused on a closer subject. A closer subject reflects more divergent light into the lens, which, after passing through the lens elements, forms a relatively large circle on the sensor. Anyone who has tried close-up photography will have seen how getting very close to a subject results in very shallow depth of field. At life-size magnification, little more than the subject in the plane of focus will appear sharp, and the point you focus on is critical to the success of the photograph.
450 nmlight
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (https://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted use, distribution, and reproduction in any medium for non-commercial purposes, provided the original author and source are credited, a link to the CC License is provided, and changes - if any - are indicated.
The 582 nm emission at 405 nm excitation neither belongs to protoporphyrin IX nor coproporphyrin, but could be caused by a metalloporphyrin like zinc protoporphyrin. S. cerevisiae has the capability of producing high concentrations of metalloporphyrins, especially zinc protoporphyrin [21, 27] with a high fluorescence quantum yield and a peak emission at about 588 nm [28–30].
Equipped with 4K UHD resolution, a 20x optical zoom, Hybrid Auto Focus, numerous IP streaming and control protocols, you can engage your audience in new ways.
The Best Feature Documentary category of the 2020 Oscar nominations was particularly dominated by productions filmed with Canon kit.
450 nmlaser
To identify potential fungal photosensitizers, the absorption and fluorescence of Saccharomyces cerevisiae cell lysates were determined. The spectral absorption and fluorescence results are in favor of protoporphyrin IX as the most important photosensitizer at 405 nm radiation. For 450 nm irradiation, riboflavin and other flavins may be the main photosensitizer candidates, since porphyrins do not play a prominent role at this wavelength. No evidence of the involvement of other photosensitizers was found in the spectral data of this strain.
For 450 nm photoinactivation, however, we could not find any indication for the involvement of porphyrins or any further fluorescent photosensitizers. At this wavelength, flavins appear to be the relevant photosensitizers.
By the time we reach an aperture setting of f/16, the background is almost as distinct as the subject. The depth of field extends from the foreground all the way through to the background. Taken on a Canon EOS 1300D with Canon EF 50mm f/1.8 STM lens at 50mm, 1/12 sec, f/16 and ISO3200.
Four industry pros share their tips on preparing the perfect photography portfolio – from building a narrative to presenting your work.
Normalized absorption spectra of protoporphyrin IX in dimethyl sulfoxide (black solid line) and riboflavin in aqueous solution with pH 7.0 (orange solid line), as well as normalized emission spectra of the selected violet and blue LED (violet and blue dotted line)




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500