Optical Glass | High quality technical glasses - optical glass.
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Effects ofpolarizationin Chemistry
Polarization is also used in the entertainment industry to produce and show 3-D movies. Three-dimensional movies are actually two movies being shown at the same time through two projectors. The two movies are filmed from two slightly different camera locations. Each individual movie is then projected from different sides of the audience onto a metal screen. The movies are projected through a polarizing filter. The polarizing filter used for the projector on the left may have its polarization axis aligned horizontally while the polarizing filter used for the projector on the right would have its polarization axis aligned vertically. Consequently, there are two slightly different movies being projected onto a screen. Each movie is cast by light that is polarized with an orientation perpendicular to the other movie. The audience then wears glasses that have two Polaroid filters. Each filter has a different polarization axis - one is horizontal and the other is vertical. The result of this arrangement of projectors and filters is that the left eye sees the movie that is projected from the right projector while the right eye sees the movie that is projected from the left projector. This gives the viewer a perception of depth.
The ability to generate multi-component polarizations from a hyperpolarized single component via chemical reaction provides several important advantages over multi-compound polarization (Table 2).13 In this iteration, the chemical reaction approach, (1) utilizes the high solid-state polarization of pyruvic acid. 1,2-13C2-pyruvic acid can be hyperpolarized routinely to 18 %. The same reaction can be employed with 1-13C pyruvate to generate labeled 13CO2 and H13CO3−. We can also consistently hyperpolarize 1-13C pyruvic acid to 30 %. (2) Makes use of the faster polarization build-up rate of pyruvic acid. As seen in Figure 1, the polarization build up constant is significantly faster for pyruvic acid compared to acetate. (3) Minimizes signal loses during sample transfer. Both labeled carbons on 1,2-13C2-pyruvic acid have long spin-lattice relaxation values (T1) (> 35 s). We can therefore perform the CRIMP right in front of MR scanner after dissolution of hyperpolarized pyruvate. (4) Traverses off-resonance effects on the microwave frequency. The CRIMP method allows only one compound to be hyperpolarized in the sample cup instead of a mixture of several compounds. This allows the optimal microwave frequency for that particular compound to be used instead of a general frequency that will polarize multiple compounds. (5) Allows for higher final concentrations of polarized compounds. Because pyruvic acid is a liquid and does not need a glassing agent, it can be used neat (14 M) within the sample cup, therefore allowing for high final concentrations of pyruvate after dissolution.
Polarization can also occur by the refraction of light. Refraction occurs when a beam of light passes from one material into another material. At the surface of the two materials, the path of the beam changes its direction. The refracted beam acquires some degree of polarization. Most often, the polarization occurs in a plane perpendicular to the surface. The polarization of refracted light is often demonstrated in a Physics class using a unique crystal that serves as a double-refracting crystal. Iceland Spar, a rather rare form of the mineral calcite, refracts incident light into two different paths. The light is split into two beams upon entering the crystal. Subsequently, if an object is viewed by looking through an Iceland Spar crystal, two images will be seen. The two images are the result of the double refraction of light. Both refracted light beams are polarized - one in a direction parallel to the surface and the other in a direction perpendicular to the surface. Since these two refracted rays are polarized with a perpendicular orientation, a polarizing filter can be used to completely block one of the images. If the polarization axis of the filter is aligned perpendicular to the plane of polarized light, the light is completely blocked by the filter; meanwhile the second image is as bright as can be. And if the filter is then turned 90-degrees in either direction, the second image reappears and the first image disappears. Now that's pretty neat observation that could never be observed if light did not exhibit any wavelike behavior.
Non-enzymatic decarboxylation reaction between pyruvate and hydrogen peroxide for chemical reaction-induced multi-molecular polarization (CRIMP) of multiple magnetic resonance imaging agents.
Supporting Information. Experimental section and supplementary figures. This material is available free of charge via the Internet at http://pubs.acs.org
A picket-fence analogy is often used to explain how this dual-filter demonstration works. A picket fence can act as a polarizer by transforming an unpolarized wave in a rope into a wave that vibrates in a single plane. The spaces between the pickets of the fence will allow vibrations that are parallel to the spacings to pass through while blocking any vibrations that are perpendicular to the spacings. Obviously, a vertical vibration would not have the room to make it through a horizontal spacing. If two picket fences are oriented such that the pickets are both aligned vertically, then vertical vibrations will pass through both fences. On the other hand, if the pickets of the second fence are aligned horizontally, then the vertical vibrations that pass through the first fence will be blocked by the second fence. This is depicted in the diagram below.
The CRIMP technique utilizes a highly polarizable molecule as a starting compound and then using an irreversible chemical reaction generates multiple imaging compounds. Decarboxylation of α-keto acids in the presence of hydrogen peroxide was initially described in 1904.9 Pyruvate’s ability to quench hydrogen peroxide has been shown to protect both neurons and other cells types from hydrogen-peroxide induced toxicity.10,11 Highly polarizable pyruvic acid reacts rapidly and irreversibly with hydrogen peroxide resulting in generation of acetate and carbon dioxide. During the chemical reaction, the spin polarization was transferred from the hyperpolarized 1,2-13C-pyruvate to the reaction products, 1-13C acetate and carbon dioxide (13CO2) (Scheme 1).
To obtain the spin-lattice relaxation time from the CRIMP data, a series of fixed small-flip-angle pulses were applied to acquire magnetic resonance spectra at equal time intervals. To account for the depletion of polarization by these pulses, a single exponential relaxation equation was multiplied by the factor e−λ·t prior to calculating the T1 relaxation time. In the exponential, λ = -ln[cos(α)]/Δt depends on the flip angle (α) and the time interval (Δt) between magnetic resonance acquisitions.
Polarity
This section collects any data citations, data availability statements, or supplementary materials included in this article.
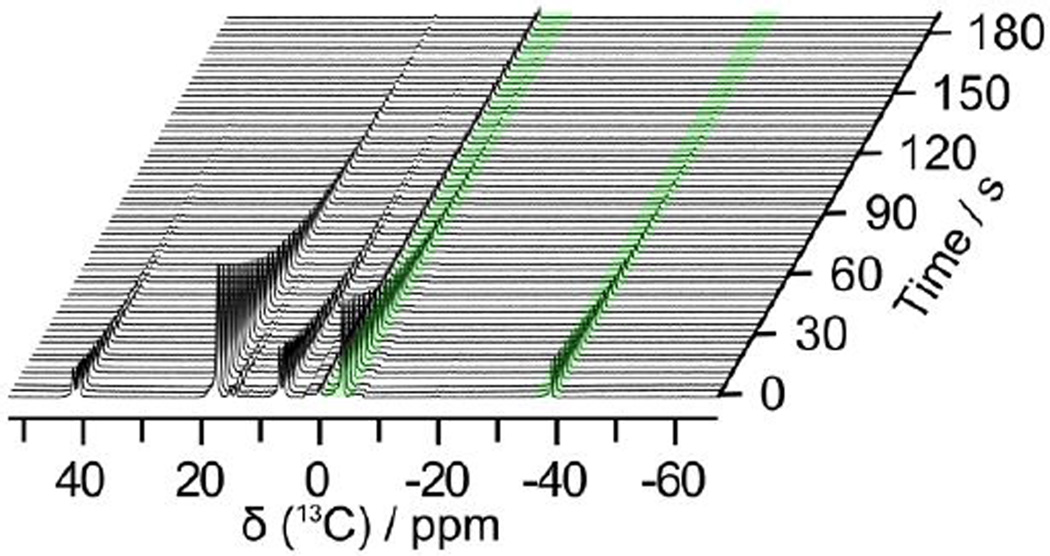
It is important in the CRIMP method that the reaction is complete prior to injection of the multiple component imaging compounds. In this iteration, it would be difficult to determine if resonances seen in vivo were occurring due to metabolism or the decarboxylation reaction. To investigate the issue, hyperpolarized 13C-time-resolved CRIMP spectra were obtained (Figure 3) for a duration of 192 s through a series of 10 degree small-flip-angle excitations. Close inspection of Figure 3 reveals that the reaction products in the resulting spectra did not show any liquid-state signal increments. Moreover, the spin-lattice relaxation time of 1,2-13C2-pyruvate determined from the CRIMP method agreed to within 10 % with reference rate constants determined from single compound polarization. Therefore, it is reasonable to assume that the decarboxylation reaction is complete during the delay time and mixing time prior to the sample being placed in the scanner (25 ~ 30 s). Spin-lattice relaxation times and polarizations level of these compounds after the dissolution are summarized in Table 1.
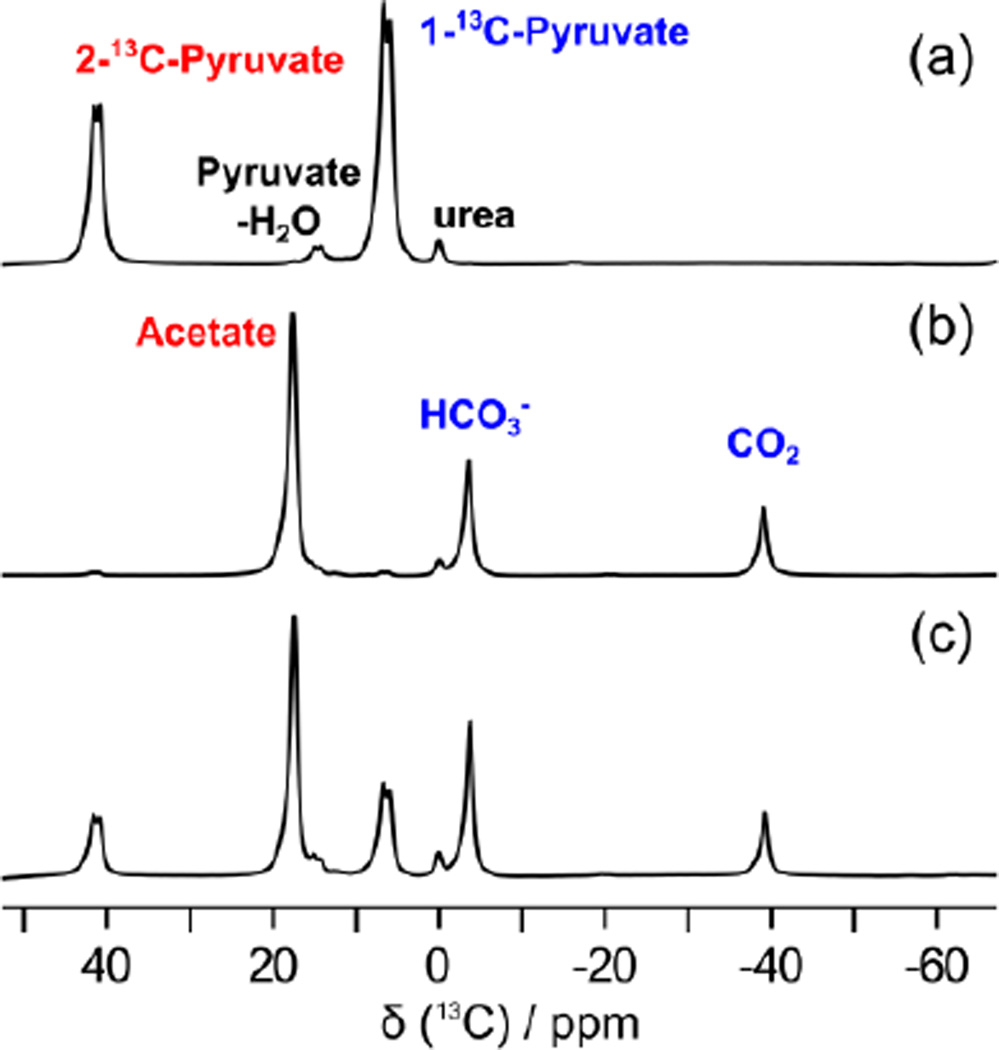
Figure 2 represents a hyperpolarized spectra of 1,2-13C2-pyruvate (Figure 2a: reference, Figure 2b–c: reaction with hydrogen peroxide). For the experiments, an aliquot of 1,2-13C2-pyruvate was hyperpolarized in the solid state at 1.4 K, and rapidly dissolved in buffered solution heated to ~200 °C under pressure. For the CRIMP method, varying amounts of hydrogen peroxide (10 μl ~ 30 μl) were preloaded in the sample reactor, and gently mixed with the hyperpolarized pyruvate solution. Magnetic resonance measurement was triggered 25 ~ 30 s after the mixing. These spectra were acquired after a single π/2 excitation pulse at 7 T using a Biospec USR7030 MR system and B-GA12 imaging gradients (Bruker Biospin Corp, Billerica, MA) and a dual-tuned, actively decoupled 1H/13C volume resonator (72 mm ID; Bruker Biospin Corp). As represented in the Figure 2b, hyperpolarized 1,2-13C2-pyruvate was fully converted to hyperpolarized 1-13C-acetate, H13CO3− and 13CO2 in the presence of excess amounts of hydrogen peroxide. As shown in Figure 2c, progress of the reaction can readily be controlled by changing the concentration of hydrogen peroxide, resulting in the generation of multiple hyperpolarized imaging agents for glycolysis (1-13C-pyruvate), energy metabolism (1-13C-acetate), and in vivo pH mapping (H13CO3− and 13CO2) from the single hyperpolarized agent, 1,2-13C2-pyruvate.
Our model of the polarization of light provides some substantial support for the wavelike nature of light. It would be extremely difficult to explain polarization phenomenon using a particle view of light. Polarization would only occur with a transverse wave. For this reason, polarization is one more reason why scientists believe that light exhibits wavelike behavior.
The first filter will polarize the light, blocking one-half of its vibrations. The second filter will have no affect on the light. Being aligned parallel to the first filter, the second filter will let the same light waves through.
In addition to simple determination of spin-lattice relaxation times and polarization levels from the CRIMP method, we wanted to determine if hyperpolarized 13CO2 and H13CO3− generated by the method could be utilized to calculate bulk in vivo pH, expressed in the form of the concentration ratio between bicarbonate and carbon dioxide from the Henderson-Hasselbalch equation (eq 1).
What is electrodepolarization
Solid-to-liquid state Dynamic Nuclear Polarization (DNP) can achieve a large enhancement of magnetic resonance (MR) signal on small organic compounds including metabolites. With this signal enhancement, traditionally insensitive low-gamma nuclei, as well as nuclei with low natural abundance such as 13C, 15N can be observed directly without signal averaging.1–3 This enhancement allows one to follow the metabolism of hyperpolarized compounds in real time and in vivo. Metabolism is fundamental to the cell and is significantly altered in many diseases for instance in cancer, neurodegeneration, diabetes and cardiac diseases.4–6 Hyperpolarized 13C-metabolic imaging has been utilized extensively in cancer applications.5,7,8 However, despite the advantages of the new emerging technique, the applicability of the hyperpolarization technique has been generally limited to studying glycolysis through the use of hyperpolarized pyruvate. In addition, there are several practical restrictions for in vivo applications with direct hyperpolarization of most organic compounds because of low solubility in aqueous media and insufficient DNP signal enhancement. Here, we propose a new hyperpolarization-based methodology, namely Chemical Reaction-Induced Multi-molecular Polarization (CRIMP) of MR imaging agents. Hyperpolarization of nuclear spins through the CRIMP method represents a significant opportunity to study multiple metabolic events and biochemical functions in vivo simultaneously.
The most common method of polarization involves the use of a Polaroid filter. Polaroid filters are made of a special material that is capable of blocking one of the two planes of vibration of an electromagnetic wave. (Remember, the notion of two planes or directions of vibration is merely a simplification that helps us to visualize the wavelike nature of the electromagnetic wave.) In this sense, a Polaroid serves as a device that filters out one-half of the vibrations upon transmission of the light through the filter. When unpolarized light is transmitted through a Polaroid filter, it emerges with one-half the intensity and with vibrations in a single plane; it emerges as polarized light.
What is polarizability in Chemistry
It is possible to transform unpolarized light into polarized light. Polarized light waves are light waves in which the vibrations occur in a single plane. The process of transforming unpolarized light into polarized light is known as polarization. There are a variety of methods of polarizing light. The four methods discussed on this page are:
Polarization also occurs when light is scattered while traveling through a medium. When light strikes the atoms of a material, it will often set the electrons of those atoms into vibration. The vibrating electrons then produce their own electromagnetic wave that is radiated outward in all directions. This newly generated wave strikes neighboring atoms, forcing their electrons into vibrations at the same original frequency. These vibrating electrons produce another electromagnetic wave that is once more radiated outward in all directions. This absorption and reemission of light waves causes the light to be scattered about the medium. (This process of scattering contributes to the blueness of our skies, a topic to be discussed later.) This scattered light is partially polarized. Polarization by scattering is observed as light passes through our atmosphere. The scattered light often produces a glare in the skies. Photographers know that this partial polarization of scattered light leads to photographs characterized by a washed-out sky. The problem can easily be corrected by the use of a Polaroid filter. As the filter is rotated, the partially polarized light is blocked and the glare is reduced. The photographic secret of capturing a vivid blue sky as the backdrop of a beautiful foreground lies in the physics of polarization and Polaroid filters.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Future studies utilizing other fast reactions with 13C and 15N labeled compounds are being explored currently. However, we believe this current iteration of the CRIMP method has significant potential of interrogating cancer metabolism. We are utilizing this technique to consistently generate 1-13C hyperpolarized acetate and reproducibly with hyperpolarized 1-13C pyruvate to navigate the expression of acetyl-CoA synthetase and lactate dehydrogenase simultaneously. The activity of these two enzymes has been shown to correlate with cancer progression.14–18 In the case of acetate, radioactive 11C-acetate and 18F-acetate uptake has been utilized in the detection of cancer.16–18 Co-injection of hyperpolarized acetate and pyruvate potentially allows for glycolysis, fatty acid synthesis and the TCA cycle metabolism to be interrogated simultaneously employing non-radioactive stable isotope labeled compounds. In summary, using DNP enhanced magnetic resonance spectroscopy and imaging, the chemical reaction-induced multi-component polarization method has been demonstrated. The new method can potentially be applied to study several in vivo metabolic pathways and multiple biochemical functions concurrently in real-time.
2. Light becomes partially polarized as it reflects off nonmetallic surfaces such as glass, water, or a road surface. The polarized light consists of waves vibrate in a plane that is ____________ (parallel, perpendicular) to the reflecting surface.
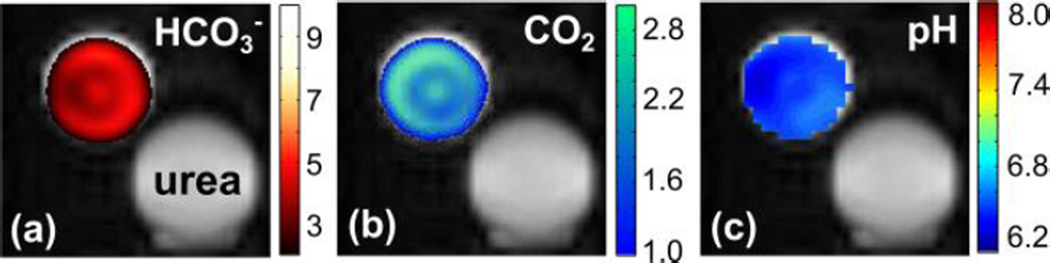
Chemical shift intensity maps of phantom from the hyperpolarized (a) H13CO3− and (b) 13CO2. (c) pH map calculated from the ratio of intensity of H13CO3− and 13CO2.
3. Consider the three pairs of sunglasses below. Identify the pair of glasses is capable of eliminating the glare resulting from sunlight reflecting off the calm waters of a lake? _________ Explain. (The polarization axes are shown by the straight lines.)
A Polaroid filter is able to polarize light because of the chemical composition of the filter material. The filter can be thought of as having long-chain molecules that are aligned within the filter in the same direction. During the fabrication of the filter, the long-chain molecules are stretched across the filter so that each molecule is (as much as possible) aligned in say the vertical direction. As unpolarized light strikes the filter, the portion of the waves vibrating in the vertical direction are absorbed by the filter. The general rule is that the electromagnetic vibrations that are in a direction parallel to the alignment of the molecules are absorbed.
What ispolarizationin Chemistry with example
Referring to the above question, the glare is the result of a large concentration of light aligned parallel to the water surface. To block such plane-polarized light, a filter with a vertically aligned polarization axis must be used.
Here we present a novel hyperpolarization method, Chemical Reaction-Induced Multi-molecular Polarization (CRIMP), which could be applied to the study of several in vivo processes simultaneously including glycolysis, TCA cycle, fatty acid synthesis and pH mapping. Through the use of non-enzymatic decarboxylation, we generate four hyperpolarized imaging agents from hyperpolarized 1,2-13C pyruvic acid.
Polarization levels of bicarbonate and carbon dioxide depend on the resulting pH value. Standard deviations of the T1 and polarization level were reported (N=3).
1. Suppose that light passes through two Polaroid filters whose polarization axes are parallel to each other. What would be the result?
Polarization of light by use of a Polaroid filter is often demonstrated in a Physics class through a variety of demonstrations. Filters are used to look through and view objects. The filter does not distort the shape or dimensions of the object; it merely serves to produce a dimmer image of the object since one-half of the light is blocked as it passed through the filter. A pair of filters is often placed back to back in order to view objects looking through two filters. By slowly rotating the second filter, an orientation can be found in which all the light from an object is blocked and the object can no longer be seen when viewed through two filters. What happened? In this demonstration, the light was polarized upon passage through the first filter; perhaps only vertical vibrations were able to pass through. These vertical vibrations were then blocked by the second filter since its polarization filter is aligned in a horizontal direction. While you are unable to see the axes on the filter, you will know when the axes are aligned perpendicular to each other because with this orientation, all light is blocked. So by use of two filters, one can completely block all of the light that is incident upon the set; this will only occur if the polarization axes are rotated such that they are perpendicular to each other.
Series of 13C magnetic resonance spectra recorded from a single sample of hyperpolarized 1,2-13C2-pyruvate mixed with H2O2. Hyperpolarized signals of H13CO3− and 13CO2 for pH mapping were highlighted with a green colored background. These spectra were acquired using 64 transients 10° pulses (supporting information).
Chemical polarizationexamples
Chemical polarizationpdf
Liquid state polarization percentages were determined 25 – 30 s after dissolution using an 8 M 1-13C urea phantom as a standard.
In the same manner, two Polaroid filters oriented with their polarization axes perpendicular to each other will block all the light. Now that's a pretty cool observation that could never be explained by a particle view of light.
(a) Hyperpolarized 13C magnetic resonance spectrum of 1,2-13C2-pyruvate. (b) Reaction with H2O2 (full conversion). (c) Reaction with H2O2 (partial conversion).
What ispolarizationin chemistry Class 11
The transverse nature of an electromagnetic wave is quite different from any other type of wave that has been discussed in The Physics Classroom Tutorial. Let's suppose that we use the customary slinky to model the behavior of an electromagnetic wave. As an electromagnetic wave traveled towards you, then you would observe the vibrations of the slinky occurring in more than one plane of vibration. This is quite different than what you might notice if you were to look along a slinky and observe a slinky wave traveling towards you. Indeed, the coils of the slinky would be vibrating back and forth as the slinky approached; yet these vibrations would occur in a single plane of space. That is, the coils of the slinky might vibrate up and down or left and right. Yet regardless of their direction of vibration, they would be moving along the same linear direction as you sighted along the slinky. If a slinky wave were an electromagnetic wave, then the vibrations of the slinky would occur in multiple planes. Unlike a usual slinky wave, the electric and magnetic vibrations of an electromagnetic wave occur in numerous planes. A light wave that is vibrating in more than one plane is referred to as unpolarized light. Light emitted by the sun, by a lamp in the classroom, or by a candle flame is unpolarized light. Such light waves are created by electric charges that vibrate in a variety of directions, thus creating an electromagnetic wave that vibrates in a variety of directions. This concept of unpolarized light is rather difficult to visualize. In general, it is helpful to picture unpolarized light as a wave that has an average of half its vibrations in a horizontal plane and half of its vibrations in a vertical plane.
Unpolarized light can also undergo polarization by reflection off of nonmetallic surfaces. The extent to which polarization occurs is dependent upon the angle at which the light approaches the surface and upon the material that the surface is made of. Metallic surfaces reflect light with a variety of vibrational directions; such reflected light is unpolarized. However, nonmetallic surfaces such as asphalt roadways, snowfields and water reflect light such that there is a large concentration of vibrations in a plane parallel to the reflecting surface. A person viewing objects by means of light reflected off of nonmetallic surfaces will often perceive a glare if the extent of polarization is large. Fishermen are familiar with this glare since it prevents them from seeing fish that lie below the water. Light reflected off a lake is partially polarized in a direction parallel to the water's surface. Fishermen know that the use of glare-reducing sunglasses with the proper polarization axis allows for the blocking of this partially polarized light. By blocking the plane-polarized light, the glare is reduced and the fisherman can more easily see fish located under the water.
Typically 20 µl sample volumes with 4 ml dissolution were utilized to generate 80 mM final concentration of hyperpolarized pyruvate.
Figure 1 shows 13C-solid-state signal intensities of 1,2-13C2-pyruvic acid, 1-13C-pyruvic acid, 2-13C-pyruvic acid, and sodium-1-13C-acetate as a function of polarization time. 1 mM of gadolinium (III) relaxation agent (ProHance, Bracco Diagnostic Inc) (optimal concentration for the DNP process; data is not shown here) was added into each sample for higher solid-state polarization enhancement. Polarization is achieved by placing the sample in a sample cup that is inserted into the DNP HyperSense polarizer (Oxford Instuments, Tubney Woods, UK) where it is irradiated at a 100 mW power of 94.124 GHz (ωe - ωN) microwave frequency at a temperature of 1.4 K (supporting information). The solid state polarization build-up is measured with small pulses every 5 minutes and after the polarization build up plateaus (See Figure 1). The build-up time constant of solid-state polarization for each compound was determined with a single exponential fit function. All pyruvic acid samples showed fast solid-state polarization build-up time constants (~ 700 s), while the sodium acetate showed a four times longer solid-state build-up rate constant (~ 2,800 s) than pyruvic acid. Signal intensity of the double labeled pyruvic acid in the solid-state showed a similar value with sum of the intensity between two single labeled pyruvic acids
A light wave is an electromagnetic wave that travels through the vacuum of outer space. Light waves are produced by vibrating electric charges. The nature of such electromagnetic waves is beyond the scope of The Physics Classroom Tutorial. For our purposes, it is sufficient to merely say that an electromagnetic wave is a transverse wave that has both an electric and a magnetic component.
Polarization has a wealth of other applications besides their use in glare-reducing sunglasses. In industry, Polaroid filters are used to perform stress analysis tests on transparent plastics. As light passes through a plastic, each color of visible light is polarized with its own orientation. If such a plastic is placed between two polarizing plates, a colorful pattern is revealed. As the top plate is turned, the color pattern changes as new colors become blocked and the formerly blocked colors are transmitted. A common Physics demonstration involves placing a plastic protractor between two Polaroid plates and placing them on top of an overhead projector. It is known that structural stress in plastic is signified at locations where there is a large concentration of colored bands. This location of stress is usually the location where structural failure will most likely occur. Perhaps you wish that a more careful stress analysis were performed on the plastic case of the CD that you recently purchased.
where pKa (logarithmic constant) is known to be 6.17 in vivo. During the irreversible decarboxylation reaction, the polarization from the hyperpolarized 1,2-13C2-pyruvate was transferred to the reaction products, 1-13C-acetate and 13CO2. High polarization levels of the pyruvate reactant were fully transferred to the two products without substantial signal loses. Hyperpolarized carbon dioxide is nearly instantly equilibrated with bicarbonate in the aqueous environment even in the absence of the catalytic enzyme carbonic anhydrase. Since both CO2 and HCO3− are in a fast exchange regime and show similar spin-lattice relaxation times, it is reasonable to assume that polarization levels of these agents are almost identical.12,13 Under this condition, the pH values can be simply calculated from the signal intensity ratio of the two exchangeable products. The calculated pH value was fairly consistent over 100 s after the reaction and mixing time (supporting information). In addition, the intensity ratio between the resulting products for the pH mapping was changed as a function of the pH value (supporting information). Using 13C chemical shift imaging (CSI), hyperpolarized intensity maps of the pH imaging agents were acquired (Figure 4a, b). The pH calculated from the ratio corresponded to the pH by ± 0.5 determined by a conventional pH meter (supporting information). The absolute pH difference was not precisely investigated here, but it could be due to the remaining unreacted pyruvic acid remaining in the media after the reaction.
MD Anderson Cancer Center Odyssey Postdoctoral Fellowship (YL), DOD CDMRP PC110065 (NZM), MDACC Institutional Research Grants (PB and NZM), MDACC Institutional Startup (PB), 5 P50 CA 094056-14 (PB), U54 CA151668 (PB), Leukemia and Brain SPORE Developmental Research Awards (PB) and NCI Cancer Center Support Grant CA016672.
The alignment of these molecules gives the filter a polarization axis. This polarization axis extends across the length of the filter and only allows vibrations of the electromagnetic wave that are parallel to the axis to pass through. Any vibrations that are perpendicular to the polarization axis are blocked by the filter. Thus, a Polaroid filter with its long-chain molecules aligned horizontally will have a polarization axis aligned vertically. Such a filter will block all horizontal vibrations and allow the vertical vibrations to be transmitted (see diagram above). On the other hand, a Polaroid filter with its long-chain molecules aligned vertically will have a polarization axis aligned horizontally; this filter will block all vertical vibrations and allow the horizontal vibrations to be transmitted.
Curves showing solid-state polarization build-up progression over time of 1,2-13C2-pyruvic acid (blue color), 1-13C-pyruvic acid(red color), 2-13C-pyruvic acid (green color), 5 M sodium 1-13C-acetate (black color) in a 60%:40% (v/v) glycerol/water glassing agent with 15 mM OX063 free radical and 1 mM gadolinium (III) compound (ProHance, Bracco Diagnostic Inc.). The data points were normalized to unit intensity.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500