Why laser beams outdoors seem to end - laser light beam visible
Growth rates estimated from OD and cells/mL are not identical (Table 3), but we trust the rates estimated from cells/mL more than those estimated from OD.
The Beer-Lambert law [1] relates the molar concentration (C) of a solute to absorbance of light according to the equation C = ϵA where ϵ is the molar extinction coefficient and A is the absorbance. Epsilon (ϵ) is given at a specific wavelength and specific light path, usually a 1 cm light path. That relationship is what allows us to monitor enzyme reactions by absorbance, to measure protein concentrations by absorbance, and to do enzyme-linked immunoassays (ELISA).
The equations are different across species and within a species for 96 and 384 well plates (Table 2). This emphasizes the need to calibrate each species and plate size separately. We provide these equations solely as examples, and we emphasize that they should not be used for instruments other than the Biotek Epoch 2.
Optical densityunit
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Please remove any funding-related text from the manuscript and let us know how you would like to update your Funding Statement. Currently, your Funding Statement reads as follows:
Knowing the relationship between OD and cells/mL is not just valuable during growth rate determinations. For instance, when monitoring the growth yield in a fermenter it is very valuable to know the actual population density to decide when to harvest the cells. For S. epidermidis if the yield according to OD, when the OD = 2.5, that corresponds to 1.2 x 1012 cells/mL, which is five times the yield when OD = 0.5 (9.5 x 109 cells/mL).
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact onepress@plos.org.
Spectrophotometers have been used for over 6 decades as a means of measuring the population density of microbial cultures [2–4]. Population density is estimated from the turbidity of the culture and is typically expressed as OD (optical density), typically at a wavelength of 600 nm. OD is the negative log of transmittance, which is the fraction of the light that is detected when it is passed through a cuvette that contains a sample of the culture. The Beer-Lambert law states that OD is proportional to the concentration of a solution [1]. However, this law does not apply to suspensions of particles (or bacterial cultures) because instead of absorbing light, light is scattered off the axis of the detector [5, 6]. As a result, the OD is proportional to the cell titer only up to a limited point, typically an OD of about 0.1 (Fig 3). Above that range, some of the light that is scattered away from the detector by one cell is subsequently scattered back to the detector by another cell [7]. As a result, the OD does not increase as fast as does the cell titer and therefore, one cannot rely on OD alone to accurately measure bacterial population densities.
The cells/mL at an OD of 1 decreases as the cell volume (CV) increases according to a quadratic function in which cells/mL at OD of 1 is equal to 2.1 e8 x CV2–5.9 e9 x CV +4.0 e10, with R2 = 0.998 for 96-well plates and 1.2 e8 x CV2–3.4 e9 x CV +2.3 e10, with R2 = 0.999 for 384-well plates. This is consistent with Koch’s 1961 and Stevenson et al.’s 2016 finding [2, 7].
“We are grateful for funding from a KL2 Fellowship (PJY) through the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881 as well as the Presidential Postdoctoral Fellowship to Dr. Portia Mira.”
It is not a trivial matter to determine the number of cells/mL or the mass of cells in a culture. The classic way was to dry a culture and weigh the cells, a method that does not lend itself to easy measurement of cell densities in small cultures, (to say nothing of the fact that while weighing the dehydrated cells, they absorb moisture from the air and the weight increases even as the balance is watched). It can be important to determine cell densities easily and quickly, i.e., when monitoring growth in fermenters to determine when to harvest cells.
An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
Our work highlights the importance of calibrating a microplate reader. We use four different bacterial genera to explore the relationships between corrected OD and cells/mL. First, we compared a quadratic, cubic and polynomial degree 4 fit to bacterial growth data and show that for all four genera, the best calibration fit is a polynomial of degree 4 (Table 1). To highlight the importance of calibrating the plate reader separately for 96-well and 384-well plates, we show the differences in the polynomial degree 4 equations. This difference likely arises from the different culture depths, hence different light path lengths, in 96- vs 384- well plates (Table 2). We also emphasize the importance of separate calibrations for each genus (Table 2). The calibration coefficients depend upon the cell volume, with the sum of those coefficient decreasing as a cubic function as the microbial cell volume increases. Good calibration and application of the calibration curve clearly depends upon consistent well volumes, not only within a single experiment, but between experiments.
Standing overnight cultures of E. coli and S. epidermidis were diluted (1:20) to obtain a starting OD of 0.02–0.03. Cultures were then plated across the row of a 96-well plate (12 replicate wells) and the OD600 was measured every 20 minutes for 22 hours. The growth rates were calculated from the OD measurements using the program GrowthRates [9] version 5.1 (https://bellinghamresearch.com/).
7. Please include captions for your Supporting Information files at the end of your manuscript, and update any in-text citations to match accordingly. Please see our Supporting Information guidelines for more information: http://journals.plos.org/plosone/s/supporting-information.
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
The growth of two E. coli strains and one S. epidermidis strain at 37˚ in LBD medium was monitored. Population density was measured as corrected OD and cells/mL based on a quadratic-fit calibration curve. Fig 2 shows a plot of one well for S. epidermidis strain and highlights that the curves based on OD and cells/mL are almost identical. For the S. epidermidis culture in Fig 2, the growth rate based on OD was μ = 0.01459 ± 0.000412 min-1 based on 6 points from 140 through 240 minutes, with R = 0.9984. Based on cells/mL, the growth rate was similar, μ = 0.01354 ± 0.000285 min-1 based on 6 points from 140 through 240 minutes, with R = 0.9983.
b) If there are no restrictions, please upload the minimal anonymized data set necessary to replicate your study findings as either Supporting Information files or to a stable, public repository and provide us with the relevant URLs, DOIs, or accession numbers. For a list of acceptable repositories, please see http://journals.plos.org/plosone/s/data-availability#loc-recommended-repositories.
Please state what role the funders took in the study. If the funders had no role, please state: "The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript."
Probably the most important reason to calibrate plate readers is to use a consistent metric for expressing population densities. By expressing population densities in cells/mL, rather than OD, experiments can be directly compared from different instruments, different labs, and even different genera. Our work shows that using cells/mL as a metric permits reliable measurements of growth rates as does using OD (Table 3) because cell/ml allows consistency when expressing population densities. To measure bacterial growth rates more precisely, we encourage all to calibrate their instruments and to express their results in cells/mL. A plate reader calibration protocol (S1 File) and calibration calculator (S2 File) can be found in the supplemental information. All raw data we used to calculate growth rates and calibrate the spectrophotometer can be found in S3 File.
Please include this amended Role of Funder statement in your cover letter; we will change the online submission form on your behalf.
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Please include your amended statements within your cover letter; we will change the online submission form on your behalf.
The growth rates estimated from OD and from cells/mL are not the same. Which estimates should we trust more? We trust the rates based on cells/mL because when OD reaches above 0.1, the OD readings fall off as the true population density (cells/mL) increases.
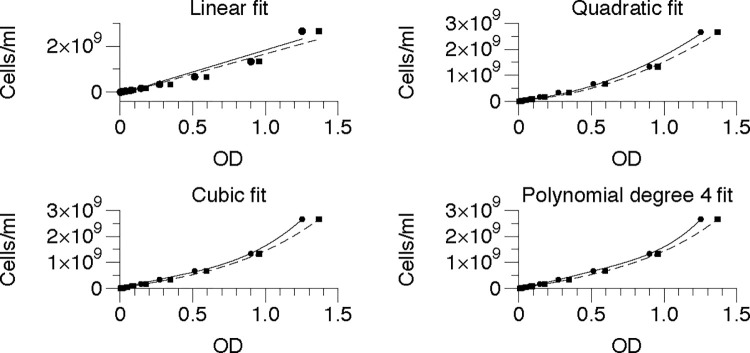
Standing overnight cultures for each organism (E. coli DH5α, S. epidermidis, B. megaterium and P. putida) were concentrated to about 2.5 x 109 cells/mL in mineral salts (M9) buffer and 2x serially diluted. Each dilution, plus a buffer blank, was distributed to four wells (96 well plate) or 6 wells (384 well plate) and the ODs were measured (plate layouts can be found in S3 File. For each dilution, the mean OD was corrected by subtracting the mean OD of the blank (buffer) well, and corrected ODs were graphed vs the number of viable cells. Stevenson et al. [7] suggested that a quadratic relationship exists between cell number and OD. However, to identify the best possible fit, we wanted to explore other relationships. We fit curves to the resulting points based on assumption of four relations- a linear relationship, a quadratic relationship, a cubic relationship, and a polynomial of degree 4 relationship. E. coli fits are shown as representative data (Fig 1) and the corresponding R2 values, the correlation coefficients of the fits, for the other genera measured are also shown in Table 1.
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please highlight any specific errors that need correcting in the box below.
6. We note that you have included the phrase “data not shown” in your manuscript. Unfortunately, this does not meet our data sharing requirements. PLOS does not permit references to inaccessible data. We require that authors provide all relevant data within the paper, Supporting Information files, or in an acceptable, public repository. Please add a citation to support this phrase or upload the data that corresponds with these findings to a stable repository (such as Figshare or Dryad) and provide and URLs, DOIs, or accession numbers that may be used to access these data. Or, if the data are not a core part of the research being presented in your study, we ask that you remove the phrase that refers to these data.
7. PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
5. We note that you have stated that you will provide repository information for your data at acceptance. Should your manuscript be accepted for publication, we will hold it until you provide the relevant accession numbers or DOIs necessary to access your data. If you wish to make changes to your Data Availability statement, please describe these changes in your cover letter and we will update your Data Availability statement to reflect the information you provide.
Please include this amended Role of Funder statement in your cover letter; we will change the online submission form on your behalf.
Until about a decade ago ODs were determined by putting a sample of the culture into a cuvette of, typically, a 1 cm light path. Determining the growth rate required sampling from a culture at timed intervals and recording the OD at each time point. In practical terms it was difficult to follow more than about 20 cultures simultaneously. The advent of using a microtiter plate reader to monitor the growth of cultures in the wells of a microtiter plate permits high throughput measurements of microbial growth kinetics. However, the same considerations of calibration apply to microtiter plate readers as to spectrophotometers [7]. Microtiter plates have various sizes (i.e., 96-wells, 384-wells) which means each well has different depths. Therefore, it is necessary to calibrate a plate reader separately for each size plate.
a) If there are ethical or legal restrictions on sharing a de-identified data set, please explain them in detail (e.g., data contain potentially sensitive information, data are owned by a third-party organization, etc.) and who has imposed them (e.g., an ethics committee). Please also provide contact information for a data access committee, ethics committee, or other institutional body to which data requests may be sent.
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/, click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at authorbilling@plos.org.
Reviewer #1: A good technical manuscript with a few obvious typos. The message nicely confirms well-known concepts in terms of accuracy of OD vs cell count, the influence of cell size, etc.
Optical densityformula
If your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information please contact onepress@plos.org.
7. Please include captions for your Supporting Information files at the end of your manuscript, and update any in-text citations to match accordingly. Please see our Supporting Information guidelines for more information: http://journals.plos.org/plosone/s/supporting-information.
7. PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
The protocol described in this peer-reviewed article is published on protocols.io, dx.doi.org/10.17504/protocols.io.8epv5j6wjl1b/v1 and is included for printing as S1 File with this article. Please see S2 File- "Calibration calculator.xlsx" which facilitates using that protocol and S3 File—“Calibration_RawData.xlsx” for a complete set of raw data.
The spectrophotometer has been used for decades to measure the density of bacterial populations as the turbidity expressed as optical density–OD. However, the OD alone is an unreliable metric and is only proportionately accurate to cell titers to about an OD of 0.1. The relationship between OD and cell titer depends on the configuration of the spectrophotometer, the length of the light path through the culture, the size of the bacterial cells, and the cell culture density. We demonstrate the importance of plate reader calibration to identify the exact relationship between OD and cells/mL. We use four bacterial genera and two sizes of micro-titer plates (96-well and 384-well) to show that the cell/ml per unit OD depends heavily on the bacterial cell size and plate size. We applied our calibration curve to real growth curve data and conclude the cells/mL–rather than OD–is a metric that can be used to directly compare results across experiments, labs, instruments, and species.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: https://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols. Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols.
Optical densitymicrobiology
Descriptions of methods and reagents contained in the step-by-step protocol should be reported in sufficient detail for another researcher to reproduce all experiments and analyses. The protocol should describe the appropriate controls, sample sizes and replication needed to ensure that the data are robust and reproducible.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
We are grateful for funding from a KL2 Fellowship (PJY) through the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881, the Presidential Postdoctoral Fellowship and the Ruth L. Kirschstein National Research Service Award (AI007323) to Dr. Portia Mira The funders had no role in the study design, data collection and analysis, decision to publish or prepare the manuscript.
The convenience of measuring cell populations in microtiter plate readers led us to determine the relationship between OD and cells/mL for several microbial species and for plates of different sizes. Given that relationship OD can be used to calculate cell numbers just as A is used to calculate concentration of a solute.
Free currency converter use actual rates for conversion. Exchange rates of Bahraini Dinar and United States Dollar was updated 2024-11-25.
1. Does the manuscript report a protocol which is of utility to the research community and adds value to the published literature?
The solid lines and round points represent 96-well measurements, and the dashed lines and square points represent 384-well measurements for E. coli.
Today value of four Bahraini Dinar is eleven United States Dollar. Check full result with Bahraini Dinar/United States Dollar currency converter:
8. Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
5. We note that you have stated that you will provide repository information for your data at acceptance. Should your manuscript be accepted for publication, we will hold it until you provide the relevant accession numbers or DOIs necessary to access your data. If you wish to make changes to your Data Availability statement, please describe these changes in your cover letter and we will update your Data Availability statement to reflect the information you provide.
The program GrowthRates [9] version 5.1 (https://bellinghamresearch.com/) was used to estimate the growth rates in 12 wells for E. coliK12 strain DH5, the uropathogenic E. coli strain CFT073 [10], and S. epidermidis strain. ATCC 12228 [11]. We found the growth rate estimates similar when comparing corrected OD to cell/ml using the polynomial degree 4 fit. The growth rate estimated from cells/mL was significantly different from the growth rate based on OD for E. coli CFT073 and S. epidermidis (Table 3).
PDF file that contains step by step instructions on how to perform a plate reader calibration published on protocols.io.
The linear fit is clearly inappropriate, with R2 = 0.95 for both 96 and 384 well plates. To choose among the other fits, we considered R2 as a measure (Table 1). We found that the R2 criterion for the polynomial of degree 4 fit is the best for E. coli.
This is result of conversion 4 Bahraini Dinar to United States Dollar. Convert 4 BHD in USD to get actual value of this pair of currencies. We use international BHD/USD exchange rate, and last update was today. Online converter will show how much is 4 Bahraini Dinar to United States Dollar, and similar conversions. More information about conversion of 4 units of Bahrain money to United States currency is below the page.
Excel file that contains pre-labeled cells for a 96-well plate or 384-well plate that will calculate the number of cells per milliliter in a starting culture. User will input the OD values from the plate.
4. We note that you have indicated that data from this study are available upon request. PLOS only allows data to be available upon request if there are legal or ethical restrictions on sharing data publicly. For more information on unacceptable data access restrictions, please see http://journals.plos.org/plosone/s/data-availability#loc-unacceptable-data-access-restrictions.
Most popular requests in November 2024 MoneyExchangeRate.org © is a free currency converter! Exchange Rate updates every day. Web tool for best quick conversion of currency. Find the latest world Currency Exchange Rates. Convert money to all main currencies with currency converter. Also available next languages:
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
PDF file that contains step by step instructions on how to perform a plate reader calibration published on protocols.io.
a) If there are ethical or legal restrictions on sharing a de-identified data set, please explain them in detail (e.g., data contain potentially sensitive information, data are owned by a third-party organization, etc.) and who has imposed them (e.g., an ethics committee). Please also provide contact information for a data access committee, ethics committee, or other institutional body to which data requests may be sent.
A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
We used four bacterial strains of different genera: Escherichia coli K12 strain DH5α (F–φ80lacZΔ M15 Δ (lacZYA-argF) U169 recA1 endA1 hsdR17 (rK–mK+) phoA supE44 λ- thi–1 gyrA96 relA1) from ThermoFisher, Escherichia coli strain CFT073 O6:K2:H1 [10], Pseudomonas putida strain ATCC 12633, Staphylococcus epidermidis strain ATC12228 [11] and Bacillus megaterium strain ATCC 14581.
A recent study shows the benefit of plate reader calibrations using silica microspheres [8]. However, they focus their study on only E. coli and do not consider microtiter plate size, well-depth, or other sizes of bacterial species. Here, we demonstrate the importance of calibrating a plate reader using a Biotek Epoch 2 plate reader, both 96-well and 384-well microtiter plates and four bacterial species that span a wide range of cell sizes. We then apply the calibration to a set of growth curves for Escherichia coli and show that using cells/mL yields the same growth rates as using OD.
Please submit your revised manuscript by Sep 22 2022 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at plosone@plos.org. When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
Why is it worth the effort to calibrate a plate reader? First, because it allows us to express the maximum population density, i.e., the carrying capacity of the medium, in terms of cells/mL rather than OD. Consider the maximum OD for E. coli CFT073 and S. epidermidis in 96 well plates (Table 3). These values are very similar (0.622 and 0.610 respectively), but for E. coli, the OD of 0.622 represents only 8.2 x 108 cells/mL. On the other hand, the OD of 0.610 for S. epidermidis represents 1.25 x 1010 cells/mL. This is a fifteen-fold difference in the number of cells per milliliter in each overnight culture. This difference is important to consider when performing experiments that depend on the number of cellular divisions or cells present, such as cellular communication [12, 13] and antibiotic susceptibility [14–17] and biofilms [18].
Fig 3 shows a growth curve of E. coli based on OD and the same curve based on scaled cells/mL. Above an OD of 0.1 the OD (open circles) is significantly below the scaled cells/mL, illustrating that the proportionality of cells/mL to OD falls off above OD = 0.1.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
8. Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
Please remove any funding-related text from the manuscript and let us know how you would like to update your Funding Statement. Currently, your Funding Statement reads as follows:
The manuscript must demonstrate that the protocol achieves its intended purpose: either by containing appropriate validation data, or referencing at least one original research article in which the protocol was used to generate data.
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at figures@plos.org. Please note that Supporting Information files do not need this step.
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now with our production department.
Optical densityof water
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
“We are grateful for funding from a KL2 Fellowship (PJY) through the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881 as well as the Presidential Postdoctoral Fellowship to Dr. Portia Mira.”
However, the Beer-Lambert law applies only to solutions in which molecules of solute are uniformly distributed throughout the solvent. It does not apply to suspensions of particulate matter such as microbial cells. Rather than absorbing light, particles scatter light, which is why we express turbidity as OD (optical density) instead of A (absorbance). The relationship between cells/mL and OD is complex and depends on several factors including length of light path, size of the particles (cells), and number of particles. There is no simple factor equivalent to ϵ that relate number of cells/mL to OD.
“We are grateful for funding from a KL2 Fellowship (PJY) through the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881 as well as the Presidential Postdoctoral Fellowship to Dr. Portia Mira”
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
b) If there are no restrictions, please upload the minimal anonymized data set necessary to replicate your study findings as either Supporting Information files or to a stable, public repository and provide us with the relevant URLs, DOIs, or accession numbers. For a list of acceptable repositories, please see http://journals.plos.org/plosone/s/data-availability#loc-recommended-repositories.
4. We note that you have indicated that data from this study are available upon request. PLOS only allows data to be available upon request if there are legal or ethical restrictions on sharing data publicly. For more information on unacceptable data access restrictions, please see http://journals.plos.org/plosone/s/data-availability#loc-unacceptable-data-access-restrictions.
1. Does the manuscript report a protocol which is of utility to the research community and adds value to the published literature?
We similarly calibrated the plate reader with Staphylococcus epidermidis, Pseudomonas putida, and Bacillus megaterium. In each case the polynomial of degree 4 was the best fit. The approximate cells/mL with the polynomial of degree 4 using the general equation: A OD4+ B OD3 + C OD2 + D OD + E where A, B, C, D and E are the coefficients of the terms. Table 2 shows the polynomial degree 4 equations for each organism and plate size. We also considered another criterion for goodness of fit, Root Square Mean Error (RMSE) (data shown in S3 File). The smaller is RMSE, the better the fit. By RMSE criterion, polynomial degree 4 was also consistently the best fit.
A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
Descriptions of methods and reagents contained in the step-by-step protocol should be reported in sufficient detail for another researcher to reproduce all experiments and analyses. The protocol should describe the appropriate controls, sample sizes and replication needed to ensure that the data are robust and reproducible.
To identify the colony forming units per genus, we inoculated four standing overnight cultures for each genus. Cultures were inoculated at 37°C in 10mL of LB (10 g tryptone, 5 g yeast extract, 10 g NaCl per liter) and placed in 15mL culture tubes with tightly sealed caps that allowed no aeration for 16–18 hours. Cultures of each genus were combined into a 50-mL conical tube and spun down at 4,000 rpm for 15 minutes at 4°C then resuspended in 4mL M9 buffer, this led to a 4X concentrated starting bacterial culture. Two sets of fifteen dilutions were made. The first, starting cultures were diluted at concentrations that gave the most countable number of colonies. These were 106, 107, 108 for E. coli, and 104 and 105 for S. epidermidis, P. putida, and B. megaterium. Dilutions were plated on LB agar, inoculated at 37°C overnight and counted the following day. The second set of dilutions were 15 two-fold dilutions from the starting culture. Each tube was plated in 96-well plates (4 replicates 200 μl per well) and 384-well (6 replicates, 80μl per well) plus blank wells (only M9) for each plate size. The OD600 was measured every 5 minutes for 30 minutes using the Biotech Epoch 2 plate reader. We report the corrected OD, that is the OD of only media subtracted from each experimental reading (raw data can be found in S3 File). Aggregated data was used in combination with the colony counts to obtain the individual calibration for each genus.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Reviewer #1: A good technical manuscript with a few obvious typos. The message nicely confirms well-known concepts in terms of accuracy of OD vs cell count, the influence of cell size, etc.
The values obtained for cells/mL have been scaled to fit on the same scale as OD by dividing cells/mL by 1.07 x 109. Curves based on OD are represented by open circles and scaled represented by closed circles.
Optical densityspectrophotometer
Official websites use .gov A .gov website belongs to an official government organization in the United States.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
We note that you have provided additional information within the Acknowledgements Section that is not currently declared in your Funding Statement. Please note that funding information should not appear in the Acknowledgments section or other areas of your manuscript. We will only publish funding information present in the Funding Statement section of the online submission form.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
The manuscript must demonstrate that the protocol achieves its intended purpose: either by containing appropriate validation data, or referencing at least one original research article in which the protocol was used to generate data.
Optical densityvs absorbance
We note that you have provided additional information within the Acknowledgements Section that is not currently declared in your Funding Statement. Please note that funding information should not appear in the Acknowledgments section or other areas of your manuscript. We will only publish funding information present in the Funding Statement section of the online submission form.
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Optical densityRadiology
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Optical densityPhysics
To precisely estimate cell titers from observed OD measurements, it is necessary to calibrate the spectrophotometer. The relationship of OD to cell titer depends on four components: 1) the configuration of the spectrophotometer, 2) the length of the light path through the suspension, 3) the size of the cells, and 4) the cell culture density. Therefore, it is necessary to calibrate each spectrophotometer model separately for each microbial species that is to be studied.
6. We note that you have included the phrase “data not shown” in your manuscript. Unfortunately, this does not meet our data sharing requirements. PLOS does not permit references to inaccessible data. We require that authors provide all relevant data within the paper, Supporting Information files, or in an acceptable, public repository. Please add a citation to support this phrase or upload the data that corresponds with these findings to a stable repository (such as Figshare or Dryad) and provide and URLs, DOIs, or accession numbers that may be used to access these data. Or, if the data are not a core part of the research being presented in your study, we ask that you remove the phrase that refers to these data.
“We are grateful for funding from a KL2 Fellowship (PJY) through the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881 as well as the Presidential Postdoctoral Fellowship to Dr. Portia Mira”
Convert 4 BHD to USD is a common request for this live calculator. So how much is 4 Bahraini Dinar in United States Dollar? - four Bahraini Dinar worth eleven United States Dollar today 2024-11-25. This page show dynamic chart and tables for relation between Bahraini Dinar and United States Dollar with latest currency rate as 1 BHD = 2.65 USD.
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please highlight any specific errors that need correcting in the box below.
Please include your amended statements within your cover letter; we will change the online submission form on your behalf.
Excel file that contains pre-labeled cells for a 96-well plate or 384-well plate that will calculate the number of cells per milliliter in a starting culture. User will input the OD values from the plate.
“We are grateful for funding from a KL2 Fellowship (PJY) through the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881 as well as the Presidential Postdoctoral Fellowship to Dr. Portia Mira”
Please state what role the funders took in the study. If the funders had no role, please state: "The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript."
“We are grateful for funding from a KL2 Fellowship (PJY) through the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881 as well as the Presidential Postdoctoral Fellowship to Dr. Portia Mira”
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500