TASK Quick Support Rods - support rods
The 5-E instructional model is an approach to teaching and learning that focuses on active engagement, inquiry-based learning, and collaboration.
Aimsof microscopepractical
Photography or image pickup with a video camera has been common in microscopy and thus a clear, sharp image over the entire field of view is increasingly required. Consequently, Plan objective lenses corrected satisfactorily for field curvature aberration are being used as the mainstream. To correct for field curvature aberration, optical design is performed so that Petzval sum becomes 0. However, this aberration correction is more difficult especially for higher-magnification objectives. (This correction is difficult to be compatible with other aberration corrections) An objective lens in which such correction is made features in general powerful concave optical components in the front-end lens group and powerful concave ones in the back-end group.
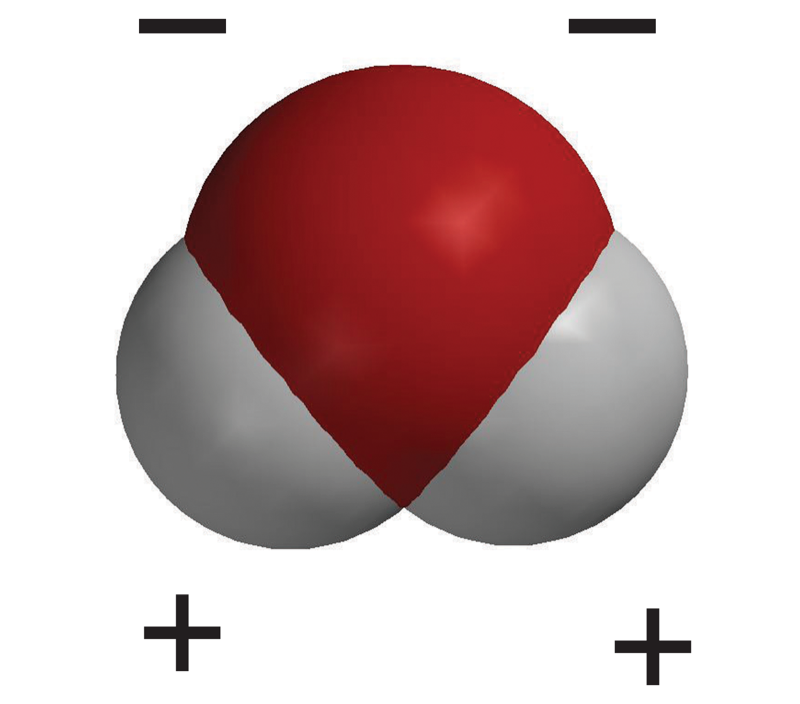
How do your Styrofoam ball models of water molecules relate to the color- coded charge density model shown in the animation?
Objectivelensmicroscopefunction
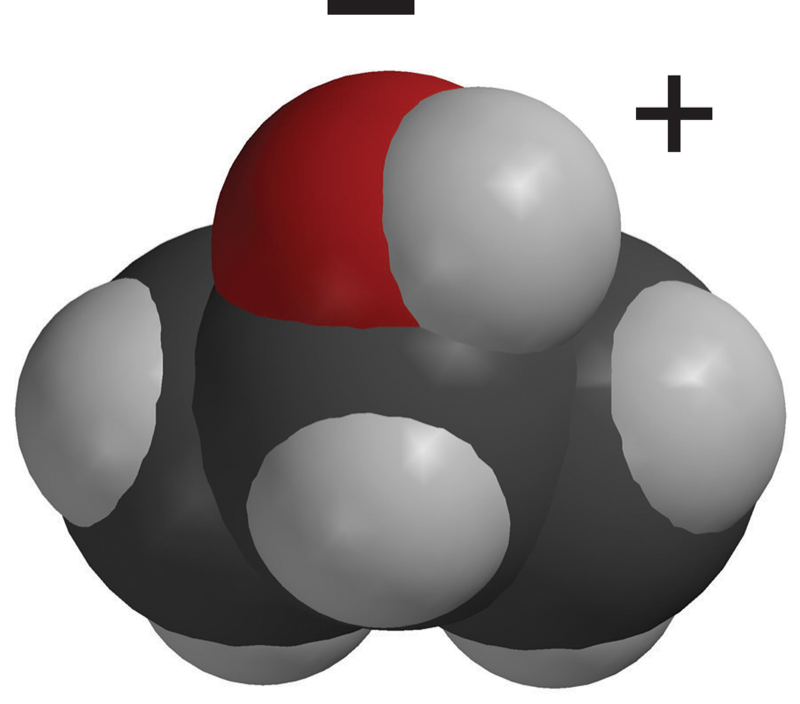
Because there are both polar and nonpolar areas on the alcohol molecule, they are somewhat less attracted to each other than water molecules are to each other. This makes it easier for alcohol molecules to come apart and move into the air as a gas. This is why alcohol evaporates faster than water.
Terms Of Use | Privacy Notice | Cookies | Cookie Settings | About Us | Imprint | Careers | Careers | Sitemap
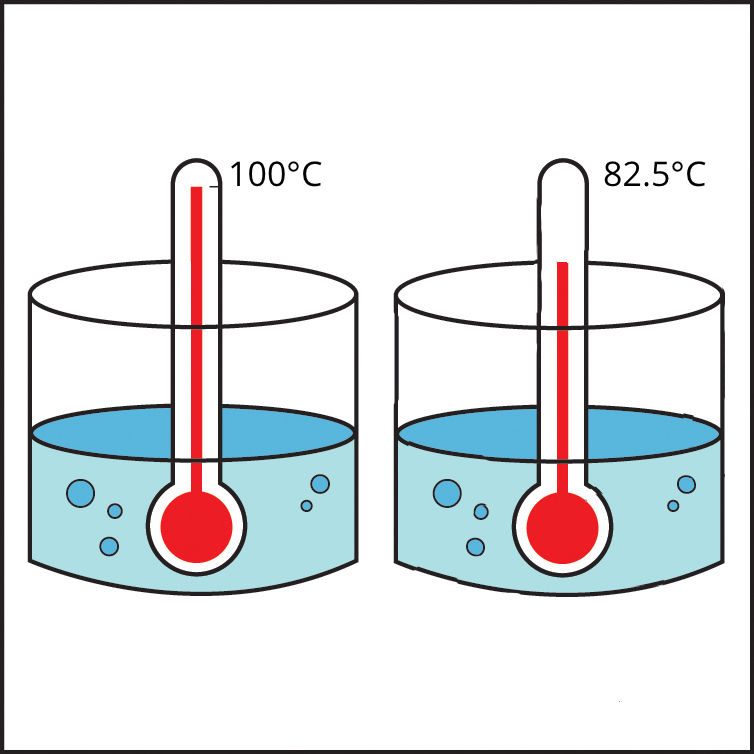
Knowing what you do about the polarity of water and alcohol, explain why alcohol boils at a lower temperature than water.
You know that water and alcohol have different characteristics because of the molecules they are made of and how these molecules interact with each other.
Axial chromatic aberration correction is divided into three levels of achromat, semiapochromat (fluorite), and apochromat according to the degree of correction. The objective lineup is divided into the popular class to high class with a gradual difference in price. An objective lens for which axial chromatic aberration correction for two colors of C ray (red: 656,3nm) and F ray (blue: 486.1nm) has been made is known as Achromat or achromatic objective. In the case of Achromat, a ray except for the above two colors (generally violet g-ray: 435.8nm) comes into focus on a plane away from the focal plane. This g ray is called a secondary spectrum. An objective lens for which chromatic aberration up to this secondary spectrum has satisfactorily been corrected is known as Apochromat or apochromatic objective. In other words, Apochromat is an objective for which the axial chromatic aberration of three colors (C, F, and g rays) has been corrected. The following figure shows the difference in chromatic aberration correction between Achromat and Apochromat by using the wavefront aberration. This figure proves that Apochromat is corrected for chromatic aberration in wider wavelength range than Achromat is.
What is thepurposeof the objectivelens inalightmicroscope
Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy.
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
Do you think a substance like water with polar molecules would evaporate faster or slower than a substance like alcohol with molecules that are not as polar?
Note: This test is fine for middle school students but there is something about the test that does not make it completely fair. There are many more water molecules in a drop of water than alcohol molecules in a drop of alcohol. The test would be more fair if the same number of water and alcohol molecules are placed on the paper towel. This requires a way to “count” molecules. Determining the number of particles in a sample is a basic concept in chemistry, but is beyond the scope of a middle school chemistry unit. Even if the same number of water and alcohol molecules were used in this activity, the alcohol would evaporate faster.
Point out that the electron cloud around the oxygen is darker than the electron cloud around the hydrogen. This shows that electrons are more attracted to the oxygen end of the molecule than the hydrogen end, making the water molecule polar.
Tell students that another way to see the difference in where the electrons are is by using the electron cloud model. Remind students that it’s impossible to know the exact location of an electron, so sometimes the regions occupied by electrons are shown as “clouds” around the nucleus in an atom or molecule.
The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. The activity sheets are formative assessments of student progress and understanding. A more formal summative assessment is included at the end of each chapter.
3. Two or more water molecules stay together because of the positive and negative parts of the molecules attracting each other.
The polar characteristic of water molecules causes them to attract each other well. The less polar alcohol molecules do not attract one another as strongly as water molecules do. It takes more energy to make water boil than it does to make alcohol boil. In other words, alcohol boils at a lower temperature than water.
Students will be able to explain, on the molecular level, what makes water a polar molecule. Students will also be able to show in a drawing that the polar nature of water can explain some of water’s interesting characteristics and help explain its evaporation rate compared to a less polar liquid.
Since the oxygen end of a water molecule is slightly negative and the hydrogen end is slightly positive, it makes sense that water molecules attract one another.
Remind students how the shared electrons in a water molecule are attracted to the protons in both the oxygen and the hydrogen atoms. These attractions hold the atoms together.
Stagemicroscopefunction
Objective lenses are roughly classified basically according to the intended purpose, microscopy method, magnification, and performance (aberration correction). Classification according to the concept of aberration correction among those items is a characteristic way of classification of microscope objectives.
Remind students that in Chapters 1 and 2, they investigated the behavior of water at different temperatures and explored the state changes of water. Many of the explanations were based on the idea that water molecules are attracted to one another. Remind students that in Chapter 4 they looked at the covalent bonding between oxygen and hydrogen, which creates the water molecule. Now students will look more closely at the details of the covalent bonds in a water molecule to understand why water molecules are attracted to one another.
Supplement in-class learning with interactive, multimedia-rich Google Forms lesson modules, perfect for reinforcing key chemistry concepts and scientific investigation skills.
The purposes of optical microscopes are broadly classified into two; "biological-use" and "industrial-use". Using this classification method, objective lenses are classified into "biological-use" objectives and "industrial-use" objectives. A common specimen in a biological use is fixed in place on the slide glass, sealing it with the cover glass from top. Since a biological-use objective lens is used for observation through this cover glass, optical design is performed in consideration of the cover glass thickness (commonly 0.17mm). Meanwhile, in an industrial use a specimen such as a metallography specimen, semiconductor wafer, and an electronic component is usually observed with nothing covered on it. An industrial-use objective lens is optically designed so as to be optimal for observation without any cover glass between the lens end and a specimen.
2. In a molecule, two or more atoms stay together because of the mutual attraction between the positively charged protons from one atom and the negatively charged electrons from the other atom. This causes the covalent or ionic bonding that holds atoms or ions together.
Students should say that they will need the same small amount of water and alcohol. These liquids should be placed at the same time on a surface like a brown paper towel so that students can tell when each liquid evaporates.
MicroscopeObjectives magnification
Students will be introduced to the idea that water has a slight positive charge at one end of the molecule and a slight negative charge at the other (a polar molecule). Students view animations, make illustrations, and use their own water molecule models to develop an understanding of how the polar nature of water molecules can help explain some important characteristics of water
Students will record their observations and answer questions about the activity on the activity sheet. The Explain It with Atoms and Molecules and Take It Further sections of the activity sheet will either be completed as a class, in groups, or individually depending on your instructions. Look at the teacher version of the activity sheet to find the questions and answers.
Explain to students that the interaction between the oxygen of one water molecule and the hydrogen of another is different than the sharing of electrons between the oxygen and the hydrogens within the water molecule itself.
Note: This video is designed to help the teacher better understand the lesson and is NOT intended to be shown to students. It includes observations and conclusions that students are meant to make on their own.
Be sure you and the students wear properly fitting goggles. Isopropyl alcohol is flammable. Keep it away from flames or spark sources. Read and follow all warnings on the label. Use in well-ventilated room. Dispose of small amounts down the drain or according to local regulations. Have students wash hands after the activity.
Meanwhile, an objective lens for which the degree of chromatic aberration correction to the secondary spectrum (g ray) is set to medium between Achromat and Apochromat is known as Semiapochromat (or Flulorite).
1. A single atom stays together because of the attraction between the positively charged protons and the negatively charged electrons.
The dark spot on the paper towel made by the alcohol will turn lighter faster than the dark spot made by the water. This indicates that the alcohol evaporates more quickly than the water.
In the optical design of microscope objectives, commonly the larger is an N.A. and the higher is a magnification, the more difficult to correct the axial chromatic aberration of a secondary spectrum. In addition to axis chromatic aberration, various aberrations and sine condition must be sufficiently corrected and therefore the correction of the secondary spectrum is far more difficult to be implemented. As the result, a higher-magnification apochromatic objective requires more pieces of lenses for aberration correction. Some objectives consist of more than 15 pieces of lenses. To correct the secondary spectrum satisfactorily, it is effective to use "anomalous dispersion glass" with less chromatic dispersion up to the secondary spectrum for the powerful convex lens among constituting lenses. The typical material of this anomalous dispersion glass is fluorite (CaF2) and has been adopted for apochromatic objectives since a long time ago, irrespective of imperfection in workability. Recently, optical glass with a property very close to the anomalous dispersion of fluorite has been developed and is being used as the mainstream in place of fluorite.
Remind students that water molecules are very polar. The strong attractions between water molecules affect water’s surface tension, boiling point, and rate of evaporation. Tell students that they will do an experiment to compare the evaporation rates of water and another liquid that isn’t as polar.
A variety of microscopy methods have been developed for optical microscopes according to intended purposes. The dedicated objective lenses to each microscopy method have been developed and are classified according to such a method. For example, "reflected darkfield objective (a circular-zone light path is applied to the periphery of an inner lens)", "Differential Interference Contrast (DIC) objective (the combination of optical properties with a DIC( Nomarski)prism is optimized by reducing lens distortions)", "fluorescence objective (the transmittance in the near-ultraviolet region is improved)", "polarization objective (lens distortions are drastically reduced)", and "phase difference objective (a phase plate is built in) are available.
Tell students that this is another model of a water molecule. In this model, color is used to show the polar areas of the water molecule. The negative area near the oxygen atom is red, and the positive area near the hydrogen atoms is blue.
Tell students that the oxygen atom attracts electrons a little more strongly than hydrogen does. So even though the electrons from each atom are attracted by both the oxygen and the hydrogen, the electrons are a bit more attracted to the oxygen. This means that electrons spend a bit more time at the oxygen end of the molecule. This makes the oxygen end of the molecule slightly negative. Since the electrons are not near the hydrogen end as much, that end is slightly positive. When a covalently bonded molecule has more electrons in one area than another, it is called a polar molecule.
Typesof microscopeobjectives
Explain that the oxygen–hydrogen (O–H) bond in the alcohol molecule is also polar. But, the carbon–hydrogen (C–H) bonds in the rest of the alcohol molecule are nonpolar. In these bonds, the electrons are shared more or less evenly.
An objective lens is the most important optical unit that determines the basic performance/function of an optical microscope To provide an optical performance/function optimal for various needs and applications (i.e. the most important performance/function for an optical microscope), a wide variety of objective lenses are available according to the purpose.
What is objectivelens inmicroscope
Terms Of Use | Privacy Notice | Cookies | Cookie Settings | About Us | Careers | Careers | Sitemap
Students made molecular models of the water molecule using Styrofoam balls and toothpicks in Chapter 2, Lesson 2. Give each student two of these water molecule models for this activity.
Microscopeparts
An optical microscope is used with multiple objectives attached to a part called revolving nosepiece. Commonly, multiple combined objectives with a different magnification are attached to this revolving nosepiece so as to smoothly change magnification from low to high only by revolving the nosepiece. Consequently, a common combination lineup is comprised from among objectives of low magnification (5x, 10x), intermediate magnification (20x, 50x), and high magnification (100x). To obtain a high resolving power particularly at high magnification among these objectives, an immersion objective for observation with a dedicated liquid with a high refractive index such as immersion oil or water charged between the lens end and a specimen is available. Ultra low magnification (1.25x, 2.5x) and ultra high magnification (150x) objectives are also available for the special use.
Point out that the water is able to stay together in these arcs because water molecules are very attracted to each other.
Remind students that the oxygen-hydrogen (O–H) bonds in water make it a polar molecule. This polarity makes water molecules attracted to each other.
Be sure students realize that no protons or electrons are gained or lost. The water molecule has a total of 10 protons and 10 electrons (8 from the oxygen atom and 1 from each of the two hydrogen atoms). Since it has the same number of protons and electrons, the water molecule is neutral.
The more-polar molecules will stick together more and will probably evaporate more slowly than less polar molecules. Less-polar molecules should evaporate faster because they are not as attracted to each other.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500