Optical Coherence Tomography (OCT) | DAIC - oct imaging system
GRIN lensfiber
Without the GRIN lens light cannot pass deep tissue, and visualization > ~1mm [42] is restricted to ex vivo. Combining GRIN lens with 1-photon [34, 35, 43, 44] and 2-photon [45] excitation techniques helps reach target areas. Additionally, commercial GRIN lenses made from thallium-containing glass and salt melts can leach toxins but bio-compatible coatings reduce toxic-effects without dropping image quality [41, 46].
Substance use disorder (SUD) involves chronic drug use [1] and can result in inabilities to meet responsibilities, brain damage, and overdose [2]. Researchers have used animal models to explore neural correlates of SUD and identify hallmarks such as “titrating” internal drug level [3], escalation [4], withdrawal [5], and drug-seeking [6]. Chronic SUD alters circuits within the mesolimbic system [7] such as nucleus accumbens (NAc) [8], amygdala [9], prefrontal cortex (PFC) [10], and hypothalamus [11]. Ideal technology for in vivo recording necessitates observation of deep brain regions, differentiation of genetically distinct cell types, longitudinal single-neuron tracking, and correlation of activity with behavior. In this short communication, we discuss how the gradient index (GRIN) lens can be implanted and used to achieve these criteria.
Grinlenses for sale
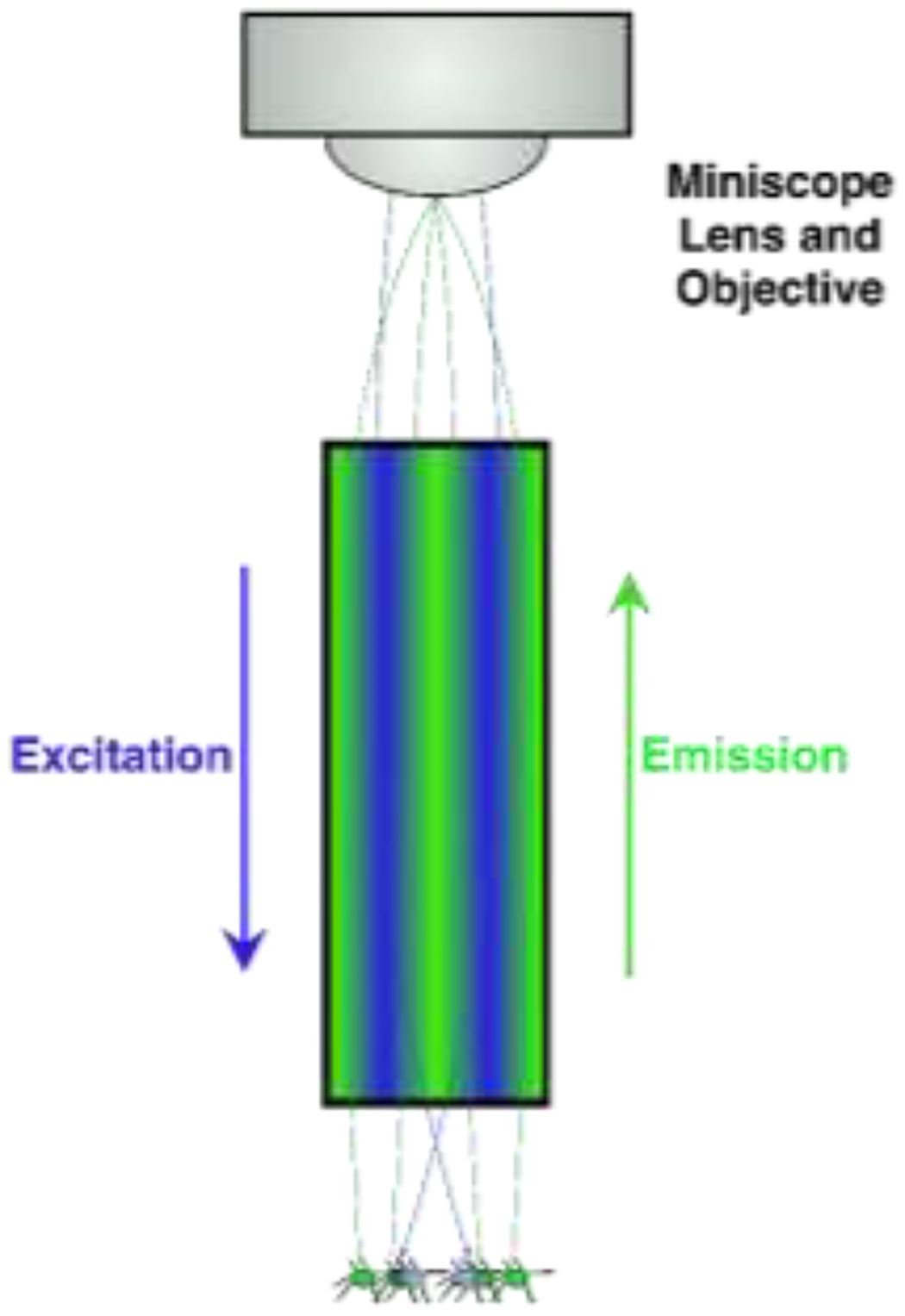
Depiction GRIN lens+ miniscope in vivo imaging. Miniscope is head mounted to a baseplate and allows for freely animal movement. A blue LED is triggered and light is reflected by a dichroic mirror into brain via the GRIN lens (see also, Figure 1b). Neurons infected with GCaMP emit green fluorescence while active in response to blue light. Green light (i.e., neural activity) is relayed back through the GRIN lens, past the dichroic mirror towards an imaging sensor and relayed offsite for analysis
This research was supported by NIH NIDA IRP. NJB, and YZ were supported by post-doctoral Fellowship from the Center on Compulsive Behaviors (CCB), National Institutes of Health. KAW was supported by NIDA IRP Scientific Director’s Fellowship for Diversity in Research (SDFDR). YL was supported by National Institute of Health (NIH) grants 5P20GM121310, R61NS115161, and UG3NS115608.
GRIN lenstheory
GRIN lenspitch
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
GRIN lensfocal length
GRIN lensInscopix
Official websites use .gov A .gov website belongs to an official government organization in the United States.
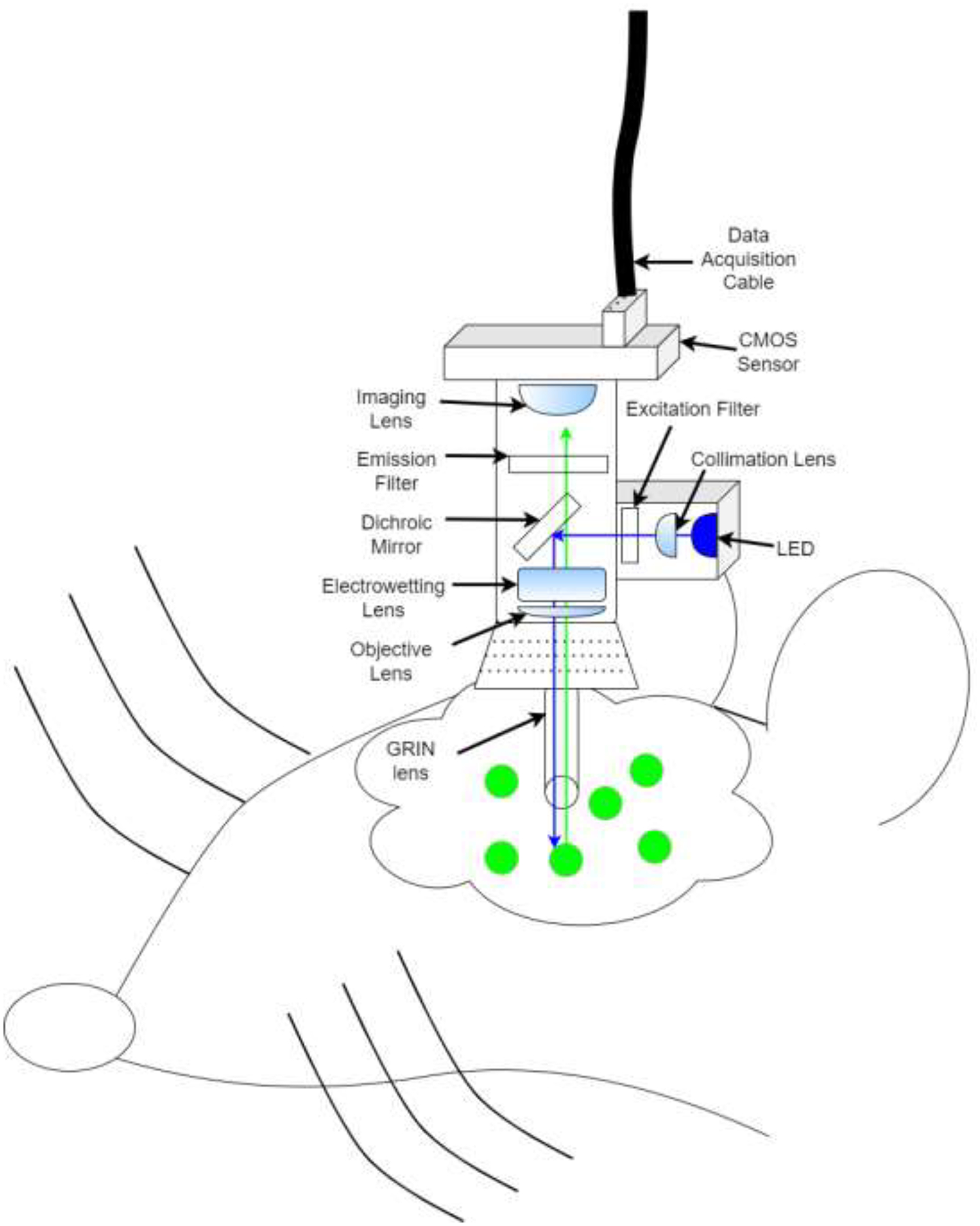
Light of a particular wavelength is transmitted from a source (e.g., LED) through a filter and dichroic mirror downward through the GRIN lens towards samples [37]). Active cells emit visible light in response to specific wavelengths and relay though the same GRIN lens towards a sensor/objective (Figure 1A) [38]. Traditional microscopy enhances signal visibility by refracting light through glass and transmits signals from glass to air by focusing light to a point [39]. The GRIN lens is crafted to refract light within one continuous glass tube to a single focal point outside of the skull (Figure 1B) [40, 41].
Grin lensprice
GRIN lensimaging
Schematic of an enlarged GRIN lens (seen in Figure 1A: Blue LED light is relayed through the GRIN lens. When neurons are active and simultaneously lit by the LED, these neurons fluoresce and produce light (green), some of which is gathered by the GRIN lens and relayed towards the objective for offsite analysis. Light is manipulated by the GRIN lens and meets at a specific focal point where the image is clear. Out-of-focus (i.e., focal length adjustment) can be via physical placement of the objectives or with an electrowetting lens (see Figure 1A) to resolve the image.
Co-corresponding authors: Nicholas James Beacher (nicholas.beacher@nih.gov), Kayden Alecsandre Washington (kayden.washington@nih.gov)
The GRIN lens enables a wide range of possibilities for future deep-brain microscopy. New techniques and tools can extract precise neurophysiological information such as sensors which detect changes in endocannabinoid [73], serotonin [74], dopamine [75], or voltage [76]. A novel “clear optically matched panoramic access channel technique (COMPACT) works by inserting a GRIN lens into an implanted tube which can be adjusted dorsoventrally to capture refracted light [77]. This method reduces GRIN lens scar tissue and allows for multiple within-subject mesolimbic targets implicated in SUD (i.e., PrL and NAc [78]). Overall, the GRIN lens provides powerful research and technological developments to ultimately help people suffering from SUD.
Substance use disorder (SUD) is associated with severe health and social consequences. Continued drug use results in alterations of circuits within the mesolimbic dopamine system. It is critical to observe longitudinal impacts of SUD on neural activity in vivo to identify SUD predispositions, develop pharmaceuticals to prevent overdose, and help people suffering from SUD. However, implicated SUD associated areas are buried in deep brain which makes in vivo observation of neural activity challenging. The gradient index (GRIN) lens can probe these regions in mice and rats. In this short communications review, we will discuss how the GRIN lens can be coupled with other technologies such as miniaturized microscopes, fiberscopes, fMRI, and optogenetics to fully explore in vivo SUD research. Particularly, GRIN lens allows in vivo observation of deep brain regions implicated in SUD, differentiation of genetically distinct neurons, examination of individual cells longitudinally, correlation of neuronal patters with SUD behavior, and manipulation of neural circuits.
Researchers have combined drug self-administration (SA) with in vivo recording technologies to correlate neural activity with behavior. Different techniques are useful for different circumstances and have pros and cons (Table 1). For instance, electrophysiology uses wires (steel, tungsten, platinum-iridium, etc.) to measure voltage changes in the extracellular environment. These act as a method to determine single-cell ‘spikes’ at high temporal resolution in deep brain regions [12, 13]. Neuronal subtypes are identified by distinct waveforms [14]. Drawbacks include data acquisition limited by channels, delicate microwires, and inability to differentiate between electrochemically similar neurons without optical manipulations [15]. Expression of immediate early gene C-fos labels large active neuronal populations and can be combined with transgenic animals [16, 17] at high spatial resolution but low temporal resolution [18] and activity does not always trigger fos expression [19]. Fast-scan cyclic voltammetry detects neurotransmission based on voltage oxidation at high temporal resolution in deep brain regions for specific neurotransmission studies [20, 21]. However, it cannot distinguish single neurons and can be clouded by high background current [22]. Fiber photometry quickly detects changes in population activity [23] using different types of sensors from deep brain regions [24] at axon terminals [25] and differentiates genetics at low cost [26] but lacks single-cell resolution [27]. The GRIN lens assists in vivo imaging in deep brain regions [28], using transgenic animals [29], and cost-effective open-source devices like miniaturized microscopes (miniscopes) [30–33] to record hundreds of individual neurons in vivo (Figure 1A) over months [34] which can be difficult to analyze. Neuron activity can be correlated with deep learning behavioral analyses [35], and optically manipulated with another LED without additional fibers [36]. However, care must be taken because physically damaging the lens or photobleaching neurons obscures activity.
Miniscope development revolutionized in vivo neuronal detection [34, 47, 48]. Originally, miniscopes used fibers to transmit light away from the organism [49] but have compartmentalized [50]. This decreased cost for behavioral microscopy and coincided with open-source miniscopes [31, 33, 50]. Miniscope and GRIN lens combination allows for single-cell visualization in deep regions such as NAc [34], and ventral tegmental area [43]. Potential GRIN lens limitations include out-of-focus fluorescence and poor optical sectioning (i.e., the ability to resolve samples embedded in tissue from noise [51], but are attenuated using an electrowetting lens to adjust the focal point [52, 53]. Motion blur can be adjusted in post-collection processes [34, 47, 48]. Major GRIN lens benefits involve targeting populations while preserving individualistic neural data. To target specific cell types transgenic animals can be bred to express cre-recombinase [54] in a multitude of cell types [55] and receptors such as μ-opioid [56], DRD1-[57], DRD2-[58], and other receptors [59].
An alternative GRIN lens approach is in conjunction with functional magnetic resonance imaging (fMRI) and a fiberscope. fMRI measures whole brain activity based on changes in cerebral blood flow [66]. A fiberscope uses calcium signaling to visualize neurons through an implanted GRIN lens, but relays towards CMOS-sensor [67, 68]. Researchers can use fiberscopes as a control for fMRI signal to understand effects of drug-associated cues across brain regions. For example, NAc and PrL are interconnected [69] and implicated in SUD [70, 71]. Following lens implants, animals would be trained for drug SA paired with a specific cue (e.g., a tone or odor). Animals would be placed in an fMRI (whole-brain) while asleep or head-fixed but awake as the animal is re-exposed to drug cues and fiberscope imaging (single-cell) would simultaneously carry signals outside of the fMRI [72]. Variations in drug-associated cue signals between the imaging techniques, PrL vs. NAc, awake vs. asleep, and correlations with relevant limbic brain regions are also possible.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
An example experiment using cre-dependent viruses (e.g., GCaMP) injected into NAc (Figure 1A). In a DRD1-iCre rat only D1- expressing cells emit fluorophores when active and illuminated with a specific frequency [34]. Multiple genetic experiments using dual-color imaging through one GRIN lens [60] inject two spectrally distinct viruses: green-emitting cre-dependent GCaMP labels D1 cells and non-cre dependent red-emitting TdTomato [61] labels all cells. Intermittently shining distinct lights onto cells induces distinct fluorescence. Post-processing distinguishes D1 cells from remaining population. Deep learning analyses correlate cell-type specific activity associated with behavioral sequences [35] and can be theoretically applied to drug use during miniscope imaging [44, 62].
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Optogenetic tools for excitation/inhibition can be integrated with the GRIN lens [36, 63]. Optogenetics use viruses containing proteins for excitation/inhibition of cells in response to specific wavelengths [64, 65]. Light optically manipulates neurons and resulting activity changes pass back to visualize activity in vivo. GRIN lens-coupled techniques can be applied in future studies to visualize neuronal progression throughout drug SA, abstinence, and relapse which have been pioneered using other in vivo techniques but can be adapted using GRIN lenses to record more neurons over longer periods of time.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500