Metalenz: Home page - new start optics
In this study we analyzed the use of microscopy in articles published in eight leading scientific journals from the pharmacology, cell biology, biochemistry, and general biomedical sciences fields (Table 1). The selected journals were British Journal of Pharmacology (BJP), Journal of Pharmacy and Pharmacology (JPP), Frontiers in Pharmacology (FP), Journal of Cell Biology (JCB), Journal of Cell Science (JCS), Cells (CEL), Journal of Biological Chemistry (JBC) and Proceedings of the National Academy of Sciences (PNAS). Since microscopy is a very dynamic field, we selected the year of 2019 to provide data related to recent articles in which newly established microscopy methods could have been used. BJP (impact factor of 7.7) is published by the British Pharmacological Society and was established in 1946. JPP (impact factor of 2.4) is the official journal of the Pharmaceutical Society of Great Britain, was established in 1870 and obtained its current title in 1949. Compared to BJP and JPP, FP (impact factor of 4.2) is a relatively new pharmacology journal (established in 2011) and is the first most cited open-access journal in the pharmacology field. JCB (impact factor of 8.8) is published by Rockefeller University Press and was established in 1962. JCS (impact factor of 4.6) was established in 1853 and it is published by The Company of Biologists. Compared to JCB and JCS, Cells (impact factor of 4.4) is a recently new journal (the first articles were published in 2012) and it is an open-access journal in the cell biology field. JBC (impact factor of 4.2) was established in 1905 and since 1925 it is published by the American Society for Biochemistry and Molecular Biology. JBC covers research in areas of biochemistry and molecular biology. PNAS (impact factor of 9.4) is the official journal of the National Academy of Sciences (USA) and one of the world’s most cited and comprehensive multidisciplinary scientific journals. We analyzed the same number of articles (200) from all the eight journals and to obtain this equal number we included all the articles published by them in 2019 and when necessary with articles from the beginning of 2020 (Table 1). We excluded from our analysis all the Reviews and Editorial Comments from these journals, and therefore, only original articles were included.
3 - Page 9: “and an image of the object of study should be almost a requirement for these studies”. It is not clear where is this fact coming from? Please check the argument, and if necessary, please provide a reference.
Mean DOFi shown by black bars in Fig. 3 is displayed horizontally in Fig. 5, because it represents a vergence interval in the object space present for each accommodative stimulus. To understand the horizontal position of each bar, we can take a look at Fig. 6, which schematically depicts the scenario for an AD of 6 D. The average accommodation response in this case was 5 D, which means that, on average, the subjects presented a 1-D lag. The corresponding DOFi bar indicates that the subjects can clearly see the object within the 6.2- to 5-D dioptric range. This means that when the object is at 6 D of AD, it is nearly at the end of the DOFi that is closest to the eye, which corresponds to about 6.2 D. The other end of the DOFi corresponds to 5 D, a plane that is paraxially conjugated with the retinal plane. Conversely, for an AD of 0 D, the DOFi range goes from −0.2 to 0.65 D. In this case, the visual system appears to use the furthermost end of this interval (−0.2 D).
A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: http://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols
Author’s response – We now included data from 8 leading scientific journal from the pharmacology, cell biology, biochemistry, and general biomedical sciences fields. The selected journals were British Journal of Pharmacology (BJP), Journal of Pharmacy and Pharmacology (JPP), Frontiers in Pharmacology (FP), Journal of Cell Biology (JCB), Journal of Cell Science (JCS), Cells (CEL), Journal of Biological Chemistry (JBC) and Proceedings of the National Academy of Sciences (PNAS); and data from 11 types of optical and electron microscopy techniques: bright field, phase contrast, differential interference contrast (DIC), polarization, conventional fluorescence, confocal fluorescence, super resolution, live cell imaging, transmission and scanning electron microscopy (TEM and SEM, respectively), and cryoEM. The whole manuscript text was revised and rewritten to strengthen our claims and analysis.
by T Mizukami · 2020 · Cited by 6 — The degree of coupling between folding and ligand binding is clearly represented by a thermodynamically meaningful parameter, %bound, and the ...
The manuscript presents a study on the use of microscopy in the fields of pharmacology and cell biology as a control. Three journals are allocated and studied, two in pharmacology and one in cell biology. Different microscopy methods were analyzed: bright field, phase contrast, DIC, polarization, conventional fluorescence, confocal, TEM and SEM. The results show different volume of usage in these two fields. In addition, common words were tested in the titles and found ‘Cell’ to be dominant in both fields. The work is interesting, and the subject is important, but there are major issues that should be corrected and added before it can be published.
1. PNAS was one of the journals that was evaluated. Note however, that PNAS is a general journal that publish articles in many subjects that are outside the scope of cell biology, or even biology. Consider how to take this into account (i.e. when quoting in page 8 the percentage of OM, the value is lower, 32%, but I believe that if this will be normalized by the percentage of bio-related publications from all publications, the number will become more similar to that of the cell-bio journals).
To test how important microscopy is to specific biomedical research fields, we decided to gather data on the use of microscopy in published articles in pharmacology, cell biology and other fields of biomedical sciences. Analysis of data obtained from published articles can be a useful tool to obtain a comprehensive view of specific research fields [22]. Our approach was to quantitatively analyze (i) the overall use of microscopy in recently published articles in pharmacology and, comparatively, in cell biology journals and other related fields, (ii) the use of different techniques of optical and electron microscopy in these articles, (iii) the correlation between the use of microscopy and cell cultures, and (iv) the differences in the vocabulary of the articles based on the relative frequency of words in their titles.
The manuscript presents a study on the use of microscopy in the fields of pharmacology and cell biology as a control. Three journals are allocated and studied, two in pharmacology and one in cell biology.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Our data support the hypothesis that the difference between the ideal and real accommodation response is mainly attributed to the presence of DOFi. That is, when accommodating to near objects, the visual system takes advantage of the full DOFi to change the refractive state of the eye by about the minimum, even if there is enough potential accommodation to improve the retinal image quality.
Different microscopy methods were analyzed: bright field, phase contrast, DIC, polarization, conventional fluorescence, confocal, TEM and SEM.
FEITO 5 - As another comment related to points 3-4, it will be interesting to have a 'negative control' that uses a subject like biochemistry or other, in order to treat the use of microscopy. The point to emphasize is that the use of a method is strongly correlated with the need.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy.
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
FALTA 3 - The usage of microscopy is compared using word cloud in the titles in section 192. The use of “cell”, “protein” and “receptor” but no further analysis is given. It does not feel that it is sufficient criteria to state “that differences in microscopy usage cannot be attributed to differences in the subject of study”. Also, there is different spellings for some words (like signaling/signalling) that can be observed in the word clouds that it is not properly explained.
First, the monochromatic ocular wavefront of seven eyes from young subjects (mean [±SD] age, 29.7 [±7.7] years) was measured at eight different accommodative demands (ADs) (from −1 to 6 diopters [D] in steps of 1 D). Then, in a second part, accommodation was paralyzed and an adaptive optics system was used to correct the aberrations of the paralyzed eye and to simulate, with the aid of an artificial pupil, the wavefront of the accommodated eye. The simulation was performed for each AD measured in the first part of the experiment. A Badal system was used to modify the stimulus vergence so as to obtain three repeated measurements of the subjective DOFi, based on the criterion of an objectionable blur.
The work is interesting, and the subject is important, but there are major issues that should be corrected and added before it can be published.
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at figures@plos.org. Please note that Supporting Information files do not need this step.
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
People with asthma often use inhalers (also called puffers) to take their medications. A spacer (also sometimes called a holding chamber) is a device that ...
What is microscopyin biology
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Fluorescence and confocal microscopy were frequently used in articles from two cell biology journals (53–70% in JCB and JCS), and less used in the journal CEL (31–36%). Conventional fluorescence (14–36%), and particularly confocal microscopy (5–12%), were infrequently used in pharmacology articles. Confocal optical microscopy is based on the suppression of out-of-focus fluorescence of three-dimensional (3D) structures. The complexity and the cost of confocal equipment varies, but they are usually quite high, and therefore this can be an important limitation on its use. Some differences in the use of confocal microscopy between cell biology and pharmacology journals could be attributed to the use of bright field in histological sections, as opposed to the optical sections obtained with confocal microscopy. Nevertheless, one must assume that pharmacological studies do not deal with 3D-structures as much as cell biology.
- The usage of microscopy is compared using word cloud in the titles in section 192. The use of “cell”, “protein” and “receptor” but no further analysis is given. It do not feel that it is sufficient criteria to state “that differences in microscopy usage cannot be attributed to differences in the subject of study “. Also there is diferent spellings for some words (like signaling/signalling) that can be observed in the word clouds that it is not properly explained.
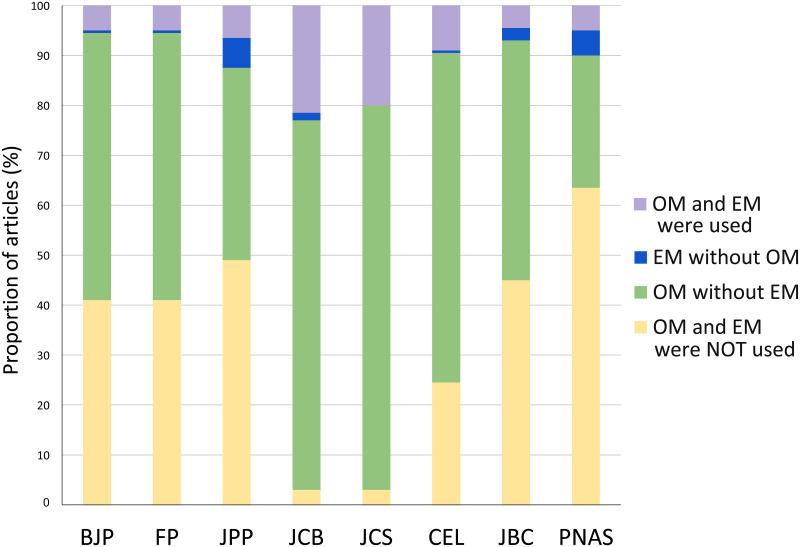
To evaluate the importance of morphological studies in different fields in biomedical sciences, we analyzed the relative use of microscopy in articles published in eight leading scientific journals from pharmacology, cell biology and other biomedical sciences fields. Our data shows (Fig 1) that microscopy was used by almost all articles from two cell biology journals (97% in JCB and JCS) and highly frequent in a new cell biology journal (75% in CEL). In a different way, pharmacology journals used microscopy in approximately half of the articles (49% in BJP and FP, 51% in JPP). We found that approximately half of the articles in a biochemistry journal used microscopy (55% in JBC), while only 36% of the articles in a multidisciplinary journal (PNAS) used microscopy. Optical microscopy (OM) was much more used than electron microscopy (EM). There are many possible reasons for the broader use of OM rather than EM: (i) because it is faster, (ii) possible to label multiple probes simultaneously, (iii) works on live cells, (iv) the biological structure is not damaged during the preparation, and (v) it can be fully quantitative. On the other side, EM is usually used where very high resolution is needed.
Considering Fig. 5, we can notice that DOFi is asymmetrically distributed with respect to the paraxial refractive state of the eye. That is, the horizontal bar is not symmetrical with respect to the accommodation response curve and the asymmetry depends on the value of the AD. Thus, for a distant object (AD = 0 D), almost all the DOFi lies in the hyperopic sense (objects closer to the eye than the stimulus). However, for an intermediate AD (1 to 3 D), the DOFi appears to be quite symmetrical about the stimulus distance, whereas for near objects, most of the DOFi expands in the myopic sense (to include objects further away from the eye than the accommodative stimulus). The DOFi’s asymmetry change during accommodation can be explained by analyzing the visual effects of the interaction between SA and defocus,34,35 as well as the change of SA with accommodation (Fig. 2B).
Fig. 5 also shows that for practically all ADs, DOFi extends from the paraxial refraction to the ideal accommodation response (1:1 dotted line). This indicates that the accommodation used by the eye is just enough to see the object correctly. It is interesting to point out that, avoiding the far and near points and their surroundings, DOFi is not needed because the eye can focus the stimulus perfectly well. For instance, Fig. 5 shows that a stimulus at an AD of 3 D can be in paraxial focus perfectly well if the eye accommodates the amount that accommodated for a stimulus placed at AD equal to 3.5 D. However, even in that case, the visual system is using most of its DOFi to accommodate the minimum.
4. Page 10 lines 222: Cryo-EM is most likely being used for basic-science studies, which explains the low-percentage use.
Depth-of-field measured for all subjects at each AD and mean value. Black bars at each AD show the intersubject mean (note that not all subjects were measured at all ADs). Error bars represent ±1 SD.
Usaj MM, Styles EB, Verster AJ, Friesen H, Boone C, Andrews BJ. High-Content Screening for Quantitative Cell Biology. Trends Cell Biol. 2016;26(8):598–611.
One of the areas in which microscopy could have made an impact is pharmacology. Imaging methods allow pharmacology researchers to address a vast number of biological questions, such as, in vivo analysis of the effects of specific drugs or molecules in a cell and/or a tissue’s morphology and physiology, nanoparticle-cell interactions, intracellular redox chemistry, mitochondrial physiology, structural determination of new drugs, among others [14–18]. Although some authors have pointed out the importance of microscopy in drug discovery [19–21], the question remains if microscopy techniques are used in pharmacology research.
TEM was not frequently used in publications (less than 20% of articles from all journals) but was the most used EM technique in all journals. We observed that the frequency of use of SEM is similar in all journals (1–5%), while TEM was much more frequently used by the cell biology journals JCB (20%) and JCS (17%). TEM and SEM are both electron microscopy methods that traditionally have been used in the biomedical sciences for detailed structural subcellular analysis of cells and tissues (TEM) and for the characterization of cell and tissue surface topography (SEM). One can argue that TEM and SEM are not widely used because they are labor and cost-intensive techniques that usually depend on specific institutional facilities. It is worth mentioning that TEM is an essential tool for the detection and analysis of the localization and ultrastructure of molecules/structures at the nanoscale level: the most up-to-date electron cryomicroscopy methods are now close to atomic resolution [24]. For example, TEM is widely used to visualize viruses in cells and tissues; and therefore, could be expected to be found more frequently in studies on the effects of specific drugs targeted to different viruses.
Our eyes project information from a three-dimensional world to a basically two-dimensional surface, corresponding to the photoreceptor plane in the retina. In theory, only one plane or surface of world can be in focus at one time. However, the eye exhibits a certain tolerance to out-of-focus images, a feature that is known as depth-of-focus (DOF). The corresponding distance range in which the objects are seen “clearly” is known as depth-of-field (DOFi). Although DOF and DOFi refer to an interval of distances or a dioptric range in the image and object space, respectively, both parameters define a similar concept and are usually interchangeable. This article will mainly refer to DOFi because it represents a more practical parameter, which can usually be determined through direct measurement.
Author’s response – As I explained above, we now included data from 8 leading scientific journal from the pharmacology, cell biology, biochemistry (as a negative control), and general biomedical sciences fields. I think that these new results have greatly improved our manuscript.
- Finally, the number of references is small and fails to introduce the previous work done in this field. The authors mention the combination of different techniques but fail to cite examples where pharmacology research has improved by the use of microscopy. For example:
Because of their varying ages, some subjects experienced blur beyond 3 D of AD. In these cases, only stimulus vergences where the subject did not experience blur were used. The number of subject analyzed for each AD was as follows: −1 to 3 D (all subjects), 4 D (six subjects), 5 D (four subjects), and 6 D (two subjects).
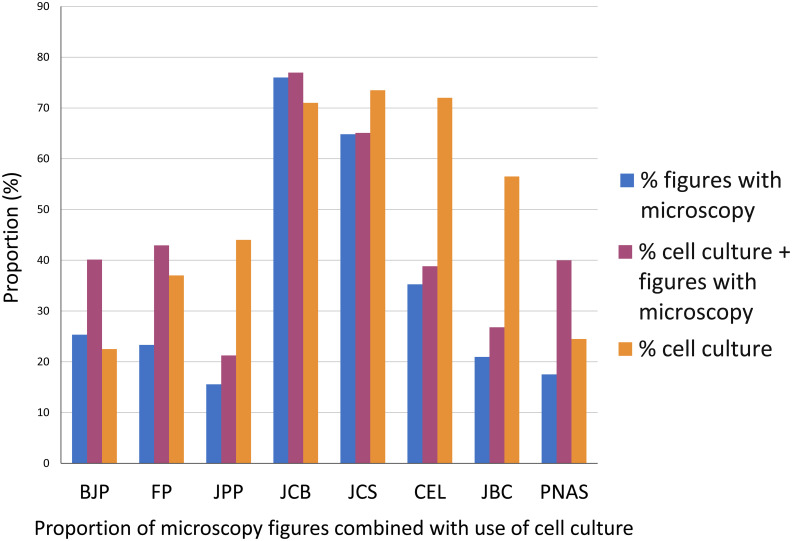
To further evaluate the impact of microscopy to each article, we analyzed the proportion of figures containing microscopy images in each article. These results showed that microscopy is usually one of the main techniques in cell biology but not in pharmacology: articles in pharmacology journals uses 16–25% of images with microscopy (Fig 4), while the cell biology journals JCB uses 76%, JCS 65% and CEL 35%. The biochemistry journal JBC uses on average 21% of microscopy in the figures of each article, while the multidisciplinary journal PNAS uses 18% of microscopy in each article. These data suggest that morphological information is more significant to research results in cell biology articles. Even though our analysis showed that nearly half of the articles from the pharmacology journals did not use any microscopy method (Fig 1), it is important to point out that a fraction of pharmacology research articles, on subjects such as biochemistry pathways and behavior, would not require microscopy. Therefore, we attempted to correlate the use of microscopy with the research theme of each article. Since the themes can be diverse, we focus on analyzing the use of cell cultures, since it is reasonable to expect that microscopes are needed for the proper visualization of cells because of their dimensions. In articles that used cell cultures, we analyzed the percentage of microscopy figures in relation to the total number of figures of the articles. The results ranged, in the pharmacology journals, from 21% in JPP to 40–43% in BJP and FP; in cell biology articles 39% in CEL to 65–77% in JCS and JCB. In JBC articles that used cell cultures, 27% of the figures used microscopy, while in PNAS articles that used cell cultures 40% of the figures used microscopy. We conclude that on average, the number of figures using microscopy in articles that use cell culture is generally higher in the cell biology field that in pharmacology. Remarkably, we obtained similar percentage of use of microscopy within total figures combined with cell culture for JPP and JBC (21 and 27%, respectively), which is indicative of a frequent use of cell culture without a corresponding emphasis in cell imaging. Interestingly, although the total use of cell culture in PNAS was low (25%), the frequency of microscopy together with cell culture (40%) was much higher than in articles that do not use cell culture (18%).
Types ofmicroscopy
6. It is important to correlate the use of microscopy with the research theme – such as tablets – tissues – drug efficiency – biomarkers and so on. A ‘comparative analysis” (line 172) similar to that, but for subjects of matter, seemed to be very important.
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
British Journal of Pharmacology (BJP), Journal of Pharmacy and Pharmacology (JPP), Frontiers in Pharmacology (FP), Journal of Cell Biology (JCB), Journal of Cell Science (JCS), Cells (CEL), Journal of Biological Chemistry (JBC) and Proceedings of the National Academy of Sciences (PNAS). The numbers at the left column of each journal refers to the number of times that each word appeared in the title of the articles (N = 200) from this journal in 2019.
The visual system takes advantage of the DOFi to change the refractive state less than necessary to form the paraxial image at the retina when it comes to focusing a near target (5 to 6 D of AD). This indicates that the main purpose of accommodation is not to maximize retinal image quality but to form one that is good enough.
Official websites use .gov A .gov website belongs to an official government organization in the United States.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
I would like to state that all listed authors qualify for authorship and agreed in the submission of the manuscript. The final version of the manuscript has been seen and approved by all coauthors. The authors declare that they have no conflict of interest.
While I believe the topic is interesting, the analyses performed fail to support the claims make by the authors. A big rewrite must be done so the authors clarify their claims and analyses. As it is, It is not written clearly enough to be accesible and new results should be included to streghen the claims. Therefore I can not recommend it for publication.
- No specific reasons are given for using such a small sample of journals (3), even if the number of scientific articles is big enough to guarantee that the analysis was not performed by hand.
A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
Fig. 3 shows that for the stimulus used, the eye’s mean DOFi amounts to about 0.9 D. Previous studies have yielded DOFi values that range between 0.8 and 1.2 D.2,25,26 In particular, Campbell2 obtained a DOFi of 0.87 D for 2 D of AD. Mordi and Ciuffreda25 reported a DOFi of about 1.28 D for an AD corresponding to the middle point of the linear interval of the accommodative response, whereas Yao et al.26 found a DOFi of 1.04 D for 1.5 D of AD.
A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
We thank Arthur Bradley and Larry N. Thibos for their comments to the interpretation of the results. This work was funded by Fundación Séneca de la Región de Murcia grant 15312/PI/10 to NL-G and by European grant ERC-2012-StG 309416-SACCO to RM-M. This study has been presented as a paper in the annual meeting of the Association for Research in Vision and Ophthalmology held in Seattle in 2013.
Fig. 2A shows that the mean (±SD) pupil diameter across all subjects was 5.70 (±0.16) mm at the paraxial FP, whereas for 6 D of AD, it dropped to 4.62 (±0.66) mm. It is worth pointing out that for the oldest subjects, R and N, the pupil size variation curve is steeper (i.e., it has a greater slope). Because these subjects have less accommodative amplitude, this curve profile may be because DOFi is greater for small pupils,2,5,31 thus compensating for the lack of accommodation. As shown in Fig. 2B, three of the seven eyes showed negative SA in the relaxed state. Although this proportion is quite high, it is nonetheless common to find subjects showing negative SA values.30,32,33 Nevertheless, on average, we can say that fourth-order SA was positive in the relaxed eye and negative in the accommodated eye as has been observed in other studies.19,21–23
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Assuming that paraxial refraction is able to correctly predict accommodative response, Fig. 5 shows that for all ADs, the visual system shows a certain lag, with the exception of the FP (0 D of AD) where the accommodation error has been forced to be zero.17 There is a simple reason behind this choice: by definition, the FP represents the minimum vergence of an object that can be clearly seen by a relaxed eye (0 D of accommodative response). Thus, following that definition, there can be no lead at 0 D of AD.
7. One of the conclusions is: “we can conclude that the pharmacology research field could gain novel horizons by including 215 new microscopy techniques in their studies.”
After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands, pending minor revisions. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
How important is it to look and study cells and tissues at the microscope in biomedical research? Which microscopy techniques are used in different fields of biomedical research? Since cells are within the micrometer scale, understanding the cellular basis of human health and disease requires the spatial resolution of microscopy [1]. Light and electron microscopy are among the major techniques used to study cellular structure and function [2]. The first microscopes were invented in 1600, and they led to the first observation of cells by Robert Hooke in 1665 [3] which in turn led to the elaboration of the Cell Theory. In the last forty years microscopy has undergone a revolution from largely qualitative observations in fixed cells to high-throughput quantitative data in live cells [4]. Today there are several different microscopy techniques to improve the visualization of fixed or live cells. Different techniques, including the light-based bright field, phase contrast, differential interference contrast (DIC), polarization, fluorescence, and confocal microscopy, and the electron-based scanning and transmission microscopy have different advantages. Both phase contrast and DIC are optical microscopy techniques used to enhance the contrast of unstained and transparent samples, including live specimens. The easiness of the phase contrast makes it a perfect match to cell cultures, while DIC achieves higher resolutions but is more labor-intensive. Polarization microscopy also enhances contrast without stain, but depends on birefringent materials, such as collagen, cellulose, myofibrils, and microtubules, and is not applicable to any molecule [5]. Fluorescence achieves very high signal to noise ratio [6], but is usually dependent on labelled antibodies or probes, or in the expression of proteins tagged with fluorescence molecules, such as green fluorescent protein (GFP) and its derivatives [7]. Confocal microscopy [8], usually based on the use of a pinhole to reject out-of-focus fluorescence, is necessary for the observation of thick fluorescent specimens, up to the size of a whole zebrafish larvae. Super-resolution microscopy methods [9] bypass the resolution limit of 0.2 um established by Ernst Abbe in the 19th century either using an interfering pattern (SR-SIM), or a de-excitation laser (STED), or can be based on the localization of fluorochromes (STORM). Emerging developments in live-cell microscopy and fluorescent labeling have begun to open unique opportunities to reveal the dynamics of biological systems with high spatio-temporal details [10, 11]. Electron microscopy achieve higher resolution than optical microscopy because the wavelength of electron is dependent on the voltage applied to the beam, and can be much smaller than the wavelength of light. The transmission electron microscopy (TEM) depends on very thin sections and contrast based on molecules, such as osmium and lead [12]. TEM uses magnetic lenses in a way similar to the transillumination of optical microscopy, while scanning electron microscopy (SEM) uses an electron beam to scan the metal-coated surface of a non-sectioned specimen. In general electron microscopy is capable of much more detail than optical microscopy, but with much more work and equipment cost. Cryogenic-electron microscopy is a powerful technique that recently emerged in structural biology, capable of delivering high-resolution density maps of macromolecular structures [13].
Norberto López-Gil Facultad de Óptica y Optometría Universidad de Murcia Campus de Espinardo 30100 Murcia Spain e-mail: norberto@um.es
What is microscopyin science
It does not seem to be a relevant conclusion, definitely not based on the study that is described. For such a statement to be true, one has to explore the type of study, the methods that are used, and compare similar studies that are done with or without microscopy.
Although accommodation leads and lags may be influenced by DOFi, there are few studies of DOFi with varying levels of accommodation, and therefore, none of the previous studies answer the key question: why is the actual accommodative response different from the ideal one for most stimulus vergences? In this work, we propose a novel methodology based on wavefront technology to provide an answer for this question.
Wavefront measurements were performed using an 850-nm-wavelength near-infrared source. Triggered by the system’s software, the deformable mirror can change its shape to correct or induce any aberration pattern expressed in terms of its Zernike decomposition up to sixth order. The target was presented by means of an internal 800- by 600-pixel microdisplay that has a maximum luminance of 30 cd/m2 measured from the observer’s position. The stimulus (Fig. 1) consisted of three lines of black letters on a white background corresponding to a logMAR (logarithm of the minimum angle of resolution) of 0.40 (top line), 0.30 (middle line), and 0.22 (lower line). Z letter in the middle line was used for the fixation during measurements and the fellow eye was occluded.
FEITO 4 - One of the conclusions is: “we can conclude that the pharmacology research field could gain novel horizons by including new microscopy techniques in their studies.” It does not seem to be a relevant conclusion, definitely not based on the study that is described. For such a statement to be true, one has to explore the type of study, the methods that are used, and compare similar studies that are done with or without microscopy. That is why point 3 is so important – The use of the technique depends on the application and the subject of study. Microscopy is definitely a great method, but its use, just like any other method, depends on the need.
Author’s response – We agree with the editor that there are many reasons for the broader use of optical microscopy rather than EM, except for the labor issue. So, we changed this sentence accordingly.
FEITO 1 - Some important microscopy methods are not included in the subjects that are tested, including Super resolution microscopy that lately immerged as a very important method. Although the issue of ‘in vivo’ and ‘live cell’ are mentioned in the introduction, these are important issues that should also be part of the study. Otherwise, we are left with partial knowledge, as it is not clear what the usage of the microscopy is.
Our analysis showed that nearly half of the articles from the pharmacology journals did not use any microscopy method, compared to the use of microscopy in almost all (97%) of the articles from the cell biology journals. We hope that our study will provide support for a critical evaluation of the impact of the use of microscopy in the pharmacology field and in biomedical sciences in general. Giving the advancement in the recent years in the microscopy field, allowing the high-resolution analysis of real-time dynamic processes in cells and tissues, we can conclude that the pharmacology research field could gain novel horizons by including new microscopy techniques in their studies.
Finally, we hypothesized that the different usage of microscopy that we observed in the journals from different fields could be related to differences in the concepts used by each field. To address this hypothesis, we would need a way of comparing the contents of each article. We therefore decide to analyze the relative use of words in the titles of the articles (S2 File). Since textual information is difficult to visualize quantitatively [25], we used the Wordle™ software to draw “word clouds” [23]. The “word clouds” give greater prominence to words that appear more frequently in the source text. Interestingly, “cell” was one of the three most frequent words found in the vocabularies from the titles of the articles from all journals (Fig 5). These results suggest that most of the articles published in these journals included analysis at the cellular level and therefore microscopy techniques would be a valuable tool for their studies. We observed that JCB and JCS showed a remarkably similar list of most frequent words: “cell”, “regulation”, “protein” and “signalling”. This word usage suggest that these articles are concerned with cellular processes at the molecular level. We also observed a somewhat similar list of most frequent words between the three pharmacology journals: “cell”, “receptor”, “inhibitor” and “cancer”, as well as similar model organisms (“mouse”, “rat” and “human”). This pattern may result from the pharmacology vocabulary itself and the focus on diseases. The remaining journals (CEL, JBC and PNAS) showed a mixed list of most frequent words.
6 - Also, it will be very helpful to organize all the relevant factual numbers in one table: number of journals, number of articles in each one. Also add N=… to the figures or figure captions.
Finally, Fig. 5 shows the mean paraxial accommodative response obtained in the first step of the methodology. Horizontal bars through each data point show the mean DOFi obtained for each target vergence shown in Fig. 3. When averaging the accommodative response, we have considered at each AD only the subjects who could see the stimulus clearly.
Fig. 4 shows the stimulus-response curve of all subjects. We have plotted the curve until the last AD for which each subject could see the stimulus clearly.
To further detail any difference in microscopy usage from different biomedical sciences fields, we analyzed in each article which OM and/or EM techniques were used (Fig 2). Overall, we found that the most frequent microscopy methods were bright field, conventional, and confocal fluorescence microscopy. In contrast, polarization microscopy was almost not used by articles in any journals, and DIC and SRes were almost only used in cell biology. Indeed, the use of polarization requires a birefringent material, while DIC and specially SRes are more labor-intensive and SRes is still a quite new and expensive microscopy technique.
An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
I submitted the manuscript entitled "How is microscopy used in pharmacology research?" (PONE-D-20-26569) for publication in PLOS ONE. The reviewers of my manuscript recommended major revision. I made the modifications in the manuscript and in its figures and I included a point-by-point response to the reviewer’s comments, which were useful to improve the data presentation and interpretation. The whole manuscript text was revised and rewritten, and new figures were added. We decided to change the title of the manuscript to “A comparative study on the use of microscopy in pharmacology and cell biology research” because of the addition of new data. I thank the Referees for their careful and appropriate analysis. The detailed corrections are as follows.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
5. In relation to the previous comment, it seems that the value of general statement “use of microscopy in pharmaceutical” is not very high, as it depends what is the actual study. The authors should refine their analysis accordingly.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
6. PLOS authors have the option to publish the peer review history of their article (what does this mean?). If published, this will include your full peer review and any attached files.
Depth-of-field of the presbyopic eye is a critical determinant of the prescribed add power or the design of contact or intraocular lenses13,14 and refractive surgery.15 It has been proposed that DOFi is responsible for some or most of the accommodative errors (lead or lag).5 Although there are some studies that have examined the origins of accommodative errors,10–12 the true origin of the accommodation error remains an unanswered question. Moreover, it has been shown quite recently that the error of accommodation can actually be a “false accommodation error” resulting from criterion differences between the subject (for subjective measurements) and the measuring device (for objective ones) when choosing the best image.16,17
Author’s response – We thank the editor for this important comment. We now analyzed the percentage of OM use in bio-related (biological sciences section of the journal) articles from PNAS. In fact, the percentage of OM in all PNAS’s articles was 32%, whereas the percentage of OM only in bio-related articles is 47%. So, we incorporated this new analysis in the new version of the manuscript.
What is microscopyused for
When increasing AD from 0 to 6 D, the mean intersubject pupil diameter and DOFi changed from 5.70 to 4.62 mm and from 0.85 ± 0.26 D to 1.07 ± 0.19 D, respectively. All subjects presented a similar DOFi for all AD (intrasubject SD never exceeded 0.23 D). Paraxial accommodation response showed a lag that increased with the AD. For the lowest (0 D) and the highest (6 D) values of AD, the refractive state of the eye was close to the nearest and furthermost ends of the DOFi, respectively.
There have been numerous studies devoted to measuring the DOFi on either side of the far point (FP) of paralyzed lens or presbyopic eyes.1,5,10,18 However, there is a lack of data regarding the DOFi as a function of the accommodative state. The reason behind this lack of data is the difficulty to accurately measure the DOFi of the accommodated eye because accommodation cannot be blocked—neither voluntarily nor involuntarily (by means of drugs) to remain at a specific desired accommodation level. The DOFi, although accommodating, can certainly differ from that of the relaxed eye because the pupil size and the aberration pattern change with accommodation.16,19–23
FEITO 2 - In 211 it is stated that cell biology and pharmacology fields have completely different uses of microscopy techniques. I do not find clear if the techniques are used for different purposed or it refers that microscopy techniques are more commonly used in cell biology.
Author’s response – We now included a new Table (supplementary Table 2) in the manuscript with all the words and their frequencies used for the generation of the word clouds.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
Solid line: mean paraxial stimulus-response curve. Dotted line: ideal response. Vertical bars represent ±1 SD of accommodation. Mean measured DOFi is represented by horizontal bars. n at the top indicates the number of subjects analyzed at each AD.
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
-It would be clarifying to add the results to which techniques are mostly use together and if there is any reason for these combinations.
2. Page 8: “Optical microscopy (OM) was much more used than electron microscopy (EM), probably because EM is more labor-intensive than OM”:
Fig. 2 shows each subject’s pupil diameter and fourth-order spherical aberration (SA) coefficient, respectively, as a function of the AD.
Here we established a procedure for the analysis of the content of articles that can be used for further similar studies in other areas and/or focusing on other aspects of research. In general, we could see several similar parameters between BJP and FP and between JBC and JCS. Interestingly, several parameters that we analyzed in JPP and CEL do not behave in a similar way to pharmacology and cell biology journals, respectively. The level of similarity between different journals of the same field should be further investigated.
Author’s response – As I explained above, we now included data from 8 leading scientific journal from the pharmacology, cell biology, biochemistry, and general biomedical sciences fields. The selected journals were British Journal of Pharmacology (BJP), Journal of Pharmacy and Pharmacology (JPP), Frontiers in Pharmacology (FP), Journal of Cell Biology (JCB), Journal of Cell Science (JCS), Cells (CEL), Journal of Biological Chemistry (JBC) and Proceedings of the National Academy of Sciences (PNAS); and data from 11 types of optical and electron microscopy techniques: bright field, phase contrast, differential interference contrast (DIC), polarization, conventional fluorescence, confocal fluorescence, super resolution, live cell imaging, transmission and scanning electron microscopy (TEM and SEM, respectively), and cryoEM. The whole manuscript text was revised and rewritten to strengthen our claims and analyses.
Image is for illustrative purposes only. Final appearance of the product may vary depending on color and accessories chosen. Depth ( ...
8. As another comment related to points 6-7, it will be interesting to have a 'negative control' that uses a subject like biochemistry ot other, in order to teat the use of microscopy. THe point to emphasize is that the use of a method is strongly correlated with the need.
A crx1 adaptive optics visual simulator (Imagine Eyes, France) was used to measure, correct, and induce optical aberrations. The simulator has a 1024-lenslet Hartmann-Shack wavefront sensor, a deformable electromagnetic mirror with 52 independent magnetic actuators as compensating element, and a Badal system. A schematic diagram of the setup is shown in Fig. 1.
All the comments of the reviewers were carefully answered as requested, but there are still minor revisions and suggestions as mentioned below.
3. It seems that two journals are not enough for the study of a discipline, and definitely a single journal for the control cell biology discipline (with much less articles relative to the pharma discipline number or articles).
For each stimulus vergence, we performed three wavefront measures and computed the average of each Zernike coefficient for the same pupil diameter, d. This diameter corresponds to the smallest of the three observed pupils rounded down to the nearest half millimeters. The spherical equivalent refractive error, M, for each AD was then computed in two different ways: one corresponding to the value that minimized the wavefront’s variance, RMS, called Zernike refraction and another one corresponding to the paraxial refraction.29 The main difference between these two values is that the first one takes into account the effects of high-order aberrations and their change during accommodation whereas the second one is insensitive to high-order aberrations.29,30
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Official websites use .gov A .gov website belongs to an official government organization in the United States.
What is microscopyin microbiology
Manual Positioning Stages Guide Motorized Linear Stage Selection Guide Motorized Goniometer & Rotary Stages Guide Multi Axis Stage & Configuration Laser ...
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Who invented microscope
The techniques analyzed were bright field (BrF), phase contrast (Pha), differential interference contrast (DIC), polarization (Pol), conventional fluorescence (Flu), confocal fluorescence (Conf), super resolution (SRes), and live cell imaging (Live); and 3 types of electron microscopy techniques: transmission (TEM), scanning (SEM) and cryo-EM (CrEM).
In the second step of the data-acquisition process, the eye’s accommodation was paralyzed by instilling one drop of cyclopentolate 1%, and a single wavefront measurement was then recorded. The Zernike coefficients were then calculated for a pupil corresponding to a diameter d (the minimum diameter each subject had when accommodating at each stimulus demand). Then, for each AD, we modified the deformable mirror to (1) offset the aberrations of the paralyzed eye and (2) add the aberration pattern measured in the first step, thus mimicking the aberrations observed when accommodating to AD. To simulate the natural pupil that the eye had at each AD, an artificial pupil with a diameter d was placed in a plane conjugated with the subject’s entrance pupil. Finally, we measured DOFi by placing the stimulus at the vergence for which we induced aberrations in the deformable mirror and then using the Badal system to change the stimulus’ vergence in the myopic and hyperopic directions in steps of 0.2 D. The subject had to indicate the maximum and minimum vergences for which the stimulus showed an objectionable blur.18 The measured vergence range was interpreted to be the DOFi that would have existed in the noncyclopleged eye for that particular AD. The ADs that were assessed ranged from 0 to 6 D, in steps of 1 D. Three measures of DOFi at each AD were taken. Depth-of-field measurements took less than 15 minutes for every subject; thus, the effect of the cyclopentolate did not change during the experiment.
Bright field was more frequently used by articles from pharmacology journals, while fluorescence was more frequently used by articles from cell biology journals, with the exception of the journal CEL, which used more bright field and less fluorescence. It is reasonable to assume that the use of bright field is mostly related to histological observations. We could speculate that pharmacology articles are more concerned with alterations in tissues caused by pharmacological treatments, while cell biology articles use fluorescence to focus on the distribution of specific molecules or organelles within cells. We cannot rule out the influence of traditional and historical methodological reasons for the different use of microscopy techniques in each field. Phase contrast microscopy was not frequently used by all the journals. This is remarkable, since several biomedical sciences papers use cell cultures, and phase contrast microscopy is a valuable technique to show cell morphology, cell culture density and many other characteristics of unlabeled live cells.
There are many reasons for the broader use of optical microscopy rather than EM, except for the labor issue. It is also because it is faster, possible to label multiple probes simultaneously, works on live cells, the structure is not damaged during the preparation, it is fully quantitative, and even though the resolution is not as good, it is still good enough to provide sub-cellular information. Most likely, it is more accurate to claim that EM is used only where very high resolution is needed, and OM cannot provide that resolution.
FEITO 2 - It seems that two journals are not enough for the study of a discipline, and definitely a single journal for the control cell biology discipline (with much less articles relative to the pharma discipline number or articles).
I submitted the manuscript entitled "A comparative study on the use of microscopy in pharmacology and cell biology research" (PONE-D-20-26569R1) for publication in PLOS ONE. After careful consideration, it was considered that the manuscript has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands, pending minor revisions. Therefore, I made the modifications in the manuscript and in its figures and I included a point-by-point response to the reviewer’s comments, which were very useful to improve the data presentation and interpretation. New figures were added. I thank the Editor for his careful and appropriate analysis.

FALTA 5 -It would be clarifying to add the results to which techniques are mostly use together and if there is any reason for these combinations.
Author’s response – We now included in the new version of the manuscript “super resolution microscopy” and “live cell/in vivo microscopy”, along with nine other microscopy methods (bright field, phase contrast, differential interference contrast, polarization, conventional fluorescence, confocal fluorescence, transmission and scanning electron microscopy, and cryoEM).
Data were collected from articles published in 2019 in the biomedical sciences journals: British Journal of Pharmacology (BJP), Journal of Pharmacy and Pharmacology (JPP), Frontiers in Pharmacology (FP), Journal of Cell Biology (JCB), Journal of Cell Science (JCS), Cells (CEL), Journal of Biological Chemistry (JBC) and Proceedings of the National Academy of Sciences (PNAS). N = 200 articles analyzed in each scientific journal.
For the analysis of the use of microscopy, we examined the following data from each article: title, abstract, methodology section, figures, figure legends and results section. We analyzed in each article the use of 8 types of optical microscopy techniques: bright field (BrF), phase contrast (Pha), differential interference contrast (DIC), polarization (Pol), conventional fluorescence (Flu), confocal fluorescence (Conf), super resolution (SRes), and live cell imaging (Live); and 3 types of electron microscopy techniques: transmission (TEM), scanning (SEM) and cryo-EM (CrEM). All the data (article’s title, DOI, use of microscopy, use of cell culture, number of figures with microscopy) was plotted in a spreadsheet and graphs were generated using Microsoft Excel™ software. We did not include some microscopy methods, such as Light Sheet, Raman, Atomic Force, Intravital, Dark Field, and High-Content Screening, because they were used by a very small number of articles.
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
During our analysis we noticed that many articles (i) did not describe at all some of the microscopy methodologies that were used, or (ii) described the microscopy methods with major errors, such as describing the use of one methodology when they used another one, and/or (iii) described the use of microscopy with important missing information on the use of microscopy methods. Remarkably, bright field and phase-contrast microscopy were the most under described microscopy techniques; most of the articles that have figures with these techniques did not mention or describe them in the methodology section. The collection of problems above described can lead to serious mistakes in the interpretation of the results presented in such papers, in the chance of an accurate repetition of these experiments and in how microscopy is perceived by researchers and their students.
Word clouds were generated using the titles of articles. The clouds give greater prominence to words that appear more frequently in the source text. Data were collected from articles published in 2019 in the biomedical sciences journals: British Journal of Pharmacology (BJP), Journal of Pharmacy and Pharmacology (JPP), Frontiers in Pharmacology (FP), Journal of Cell Biology (JCB), Journal of Cell Science (JCS), Cells (CELL), Journal of Biological Chemistry (JBC) and Proceedings of the National Academy of Sciences (PNAS). N = 200 articles analyzed in each scientific journal.
Key Words: depth-of-focus, depth-of-field, accommodation, aberrations, spherical aberration, adaptive optics, accommodative lag, pupil, accommodation response
Norberto López-GilFacultad de Óptica y OptometríaUniversidad de MurciaCampus de Espinardo30100 MurciaSpaine-mail: norberto@um.es
Microscopy is the main technique to visualize and study the structure and function of cells. The impact of optical and electron microscopy techniques is enormous in all fields of biomedical research. It is possible that different research areas rely on microscopy in diverse ways. Here, we analyzed comparatively the use of microscopy in pharmacology and cell biology, among other biomedical sciences fields. We collected data from articles published in several major journals in these fields. We analyzed the frequency of use of different optical and electron microscopy techniques: bright field, phase contrast, differential interference contrast, polarization, conventional fluorescence, confocal, live cell imaging, super resolution, transmission and scanning electron microscopy, and cryoelectron microscopy. Our analysis showed that the use of microscopy has a distinctive pattern in each research area, and that nearly half of the articles from pharmacology journals did not use any microscopy method, compared to the use of microscopy in almost all the articles from cell biology journals. The most frequent microscopy methods in all the journals in all areas were bright field and fluorescence (conventional and confocal). Again, the pattern of use was different: while the most used microscopy methods in pharmacology were bright field and conventional fluorescence, in cell biology the most used methods were conventional and confocal fluorescence, and live cell imaging. We observed that the combination of different microscopy techniques was more frequent in cell biology, with up to 6 methods in the same article. To correlate the use of microscopy with the research theme of each article, we analyzed the proportion of microscopy figures with the use of cell culture. We analyzed comparatively the vocabulary of each biomedical sciences field, by the identification of the most frequent words in the articles. The collection of data described here shows a vast difference in the use of microscopy among different fields of biomedical sciences. The data presented here could be valuable in other scientific and educational contexts.
FEITO 1 - This work studies the use of microscopy in pharmacological research. To do this, the authors propose to do a bibliometrics analysis to review scientific publications during the year 2019 on two pharmacology journals (BJP and FP) and one cell biology journal for control. The following data is recollected
Please submit your revised manuscript by Jan 14 2021 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at plosone@plos.org. When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Objective (at the specimen ) times the ocular (lens at the eye). In example: 10x times 15x = 150x magnification. There are several inexpensive ...
Data acquisition was carried out in two separate steps. First, a wavefront was measured as a function of the accommodative stimulus by varying the object’s vergence from 1 D beyond FP up to 6 D of accommodation in steps of 1 D; that is, accommodative demands (ADs) ranging from −1 to 6 D were tested. After each change of stimulus vergence, the subjects were given 1.5 seconds to accommodate before the wavefront was obtained. Subject N was an exception, who required 3 seconds to feel comfortable. Wavefront measurement required 33 milliseconds. We urged the subjects to try to maintain the stimulus sharp at all times.
Author’s response – We now revised the whole manuscript, including the Conclusions, to incorporate new data on the use of cell cultures together with microscopy. In this way we tried to separate the articles in two types: research at the cellular level or at the whole animal/human level.
5. I may have missed it, but it will be useful to have a table of words usage, the source for the word-cloud images. I t can be a supplement table.
As a rule of thumb, a performance optimised optical design will require the physical lens to be as long as its focal length. So, if you have a requirement for a ...
Several authors have used an optical system in which the subject focuses his or her attention on a stimulus located at a given distance, while another stimulus close to it can be moved voluntarily by the subject until its image is no longer clear and sharp, that is, until it falls out of their DOFi.2 However, it is quite difficult to perform this task with accuracy, because VA decreases very rapidly with stimulus eccentricity.24 As far as we are aware, there are only three studies that measured DOFi for objects located between the far and near points.2,25,26 Campbell2 used a stimulus made up of a vertical row of three dark discs. The vergence was set during the experiment to a fixed −2 diopter (D) value and it was used at a fixation point that presumably led to a steady accommodation state. Two plates, one with a single disc on the left of the vertical row and another one having the disc on the right side, were moved by the subject along an optical bench so as to find the distance range in which both discs could be seen clear simultaneously. In 1998, Mordi and Ciuffreda25 used a similar technique to study a large population having a wider age range. For each subject, they measured the subjective DOFi in the middle of the linear part of the stimulus-response curve. Moreover, in 2010, Yao et al.26 performed objective and subjective measures of DOFi for 0, −1.5, and −4 D stimulus vergence under the hypothesis that the accommodative and perceptual systems have different abilities to detect blur. The system used was similar to that from Campbell, but only measured the distal half of the DOFi. Therefore, estimating the total DOFi with this approach assumes that DOFi is symmetric in the object space.5
Author’s response – We revised all the words present in the word clouds to resolve the different spellings problem (American versus English language). We also reviewed the word cloud section.
4. For the pharmaceutical analysis, it is important to know what kind of study is described in the manuscript. One can assume that studying histological sections will necessitate microscopy, while studying drug tablets not necessarily requires microscopy. Mixing these together may bias the results and certainly the conclusions.
That is why point 6 is so important – The use of the technique depends on the application and the subject of study. Microscopy is definitely a great method, but its use, just like any other method, depends on the need.
We hope that the modifications made in the new version of the manuscript have properly addressed the criticism and suggestions made by the referee, and that the improvements made in the manuscript will be sufficient for its publication in PLOS ONE.
Next, we asked if the different microscopy techniques were used in combination or isolated. Therefore, we plotted the number of microscopy techniques used concomitantly in each article. As described before, nearly 50% of the articles published in all pharmacology journals (BJP, FP and JPP), and in the biochemistry journal JBC employed no microscopy methods (Fig 3). Remarkably, 64% of the articles from PNAS did not use any microscopy, which could be explained by the variety of research areas it covers since it is a multidisciplinary journal. JCB and JCS articles more frequently combine 2 microscopy techniques, while CEL more frequently uses only a single microscopy technique. The cell biology journals used combinations of 2 to even 6 microscopy methods in the same article. No article from any journal used more than 6 microscopy methods from the total of 11 methods that we analyzed. It is worth mentioning that these microscopy techniques have different advantages and may complement each other, and therefore their combined use could improve the article and should be stimulated. S1 File shows the frequency of each of the combinations of microscopy techniques that were used simultaneously in the articles. The combination use of 6 techniques were used in only 2 articles (from JCB) and these were: BrF + DIC + Flu + Conf + Live + SEM, and BrF + Flu + Conf + SRes + Live + TEM. The combined use of 5 techniques were found in 15 articles and the most frequent one was BrF + Pha + Flu + Conf + Live. The concomitant use of 4 techniques were found in 37 articles and the most frequent one was DIC + Flu + Conf + Live. The use of 3 techniques was present in 41 articles and the most frequent ones were BrF + Flu + Conf, BrF + Pha + Flu, and Conf + SRes + Live. Finally, the combination of two techniques were detected in 28 articles and the most common ones were BrF + Flu, BrF + Conf, Flu + Conf, Conf + Live, Pha + Flu, Conf + TEM, and Flu + Live. Interestingly, although DIC and SRes were not frequently used techniques they were always used in combination with other techniques.
The relaxed eye usually shows positive SA (Fig. 2B), and Thibos et al. have shown that in the presence of a positive SA, a hyperopic plane (negative defocus) with respect to the paraxial image will show a higher-contrast image, but the letters of the object will be more difficult to read owing to the changes in the phase transfer function, producing contrast reversal phenomena.34,35 However, a myopic blur (positive defocus) will decrease the contrast of the object without creating phase reversals, allowing a better readability of high-contrast letters. This asymmetry in the effects of defocus may explain why most of the DOFi at AD equal to 0 D expands to higher values of AD or negative defocus (Fig. 5). On the contrary, for SA less than 0 (usually in the accommodated eye, Fig. 2B), a similar contrast reversal phenomenon will occur with positive defocus and better legibility with negative defocus. This explains why most of the DOFi for high accommodation values expands toward lower values of AD (Fig. 5). For SA equal to 0, around AD equal to 3 D (Fig. 2B), a symmetry in the DOFi can be expected, as it was indeed obtained (Fig. 5).
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/. PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at figures@plos.org. Please note that Supporting Information files do not need this step.
An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
FEITO 3 - For the pharmaceutical analysis, it is important to know what kind of study is described in the manuscript. One can assume that studying histological sections will necessitate microscopy, while studying drug tablets not necessarily requires microscopy. Mixing these together may bias the results and certainly the conclusions. In relation to the previous comment, it seems that the value of general statement “use of microscopy in pharmaceutical” is not very high, as it depends what is the actual study. The authors should refine their analysis accordingly. It is important to correlate the use of microscopy with the research theme – such as tablets – tissues – drug efficiency – biomarkers and so on. A ‘comparative analysis” (line 172) similar to that, but for subjects of matter, seemed to be very important.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: http://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols
Author’s response – We now included a new Table in the manuscript containing all the study’s relevant factual numbers (number of journals, journal’s abbreviations, number of articles in each one) and we also added to all the figure captions the number of articles (N) used for each journal.
British Journal of Pharmacology (BJP), Journal of Pharmacy and Pharmacology (JPP), Frontiers in Pharmacology (FP), Journal of Cell Biology (JCB), Journal of Cell Science (JCS), Cells (CEL), Journal of Biological Chemistry (JBC) and Proceedings of the National Academy of Sciences (PNAS). The numbers at the left column of each journal refers to the number of times that each word appeared in the title of the articles (N = 200) from this journal in 2019.
Stimulus-response curve of all subjects. We have plotted the curves until the last AD for which the subject could see the stimulus clear. The black line shows the mean response.
Analysis of the presence of optical (OM) and electron (EM) microscopy methodologies in all articles published in 2019 in the journals: British Journal of Pharmacology (BJP), Journal of Pharmacy and Pharmacology (JPP), Frontiers in Pharmacology (FP), Journal of Cell Biology (JCB), Journal of Cell Science (JCS), Cells (CEL), Journal of Biological Chemistry (JBC) and Proceedings of the National Academy of Sciences (PNAS). N = 200 articles analyzed in each scientific journal.
What is microscopypdf
Magnifies the specimen for observation, located at the top of the microscope. 9. Arm. Connects the microscope head ...
The results show different volume of usage in these two fields. In addition, common words were tested in the titles and found ‘Cell’ to be dominant in both fields.
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) number 407331/2018-2 to C.M., and Fundação Carlos Chagas Filho de Apoio à Pesquisa do Estado do Rio de Janeiro (FAPERJ) number E-26/202.920/2019 to C.M. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Parts of microscope
Choose from our selection of M3 set screws, including cup-point set screws, nonmarring set screws, and more. In stock and ready to ship.
We analyzed in each article the use of 8 types of optical microscopy techniques: bright field (BrF), phase contrast (Pha), differential interference contrast (DIC), polarization (Pol), conventional fluorescence (Flu), confocal fluorescence (Conf), super resolution (SRes), and live cell imaging (Live); and 3 types of electron microscopy techniques: transmission (TEM), scanning (SEM) and cryo-EM (CrEM). Data were collected from articles published in 2019 in the biomedical sciences journals: British Journal of Pharmacology (BJP), Journal of Pharmacy and Pharmacology (JPP), Frontiers in Pharmacology (FP), Journal of Cell Biology (JCB), Journal of Cell Science (JCS), Cells (CEL), Journal of Biological Chemistry (JBC) and Proceedings of the National Academy of Sciences (PNAS). N = 200 articles analyzed in each scientific journal.
Please submit your revised manuscript by Feb 18 2021 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at plosone@plos.org. When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now with our production department.
This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Author’s response – The Results section of the new version of the manuscript was restructured to make our results easily understandable.
Author’s response – We now included a new supplementary figure (Table 1) with the information related to which techniques are mostly used together and we discuss some of these combinations.
This study comprises a total of seven eyes—five right and two left ones—from seven trained subjects. The subjects’ age ranged from 22 to 44 years, with a mean (±SD) value of 29.7 (±7.7) years. Subjects in this study were staff from the University of Valencia and the University of Murcia. Most subjects had participated in previous accommodation experiments and all of them participated in a short training session to establish a consistent criterion for “objectionable blur.” The research followed the tenets of the Declaration of Helsinki and informed consent was obtained from subjects after they heard a detailed explanation of the study.
Schematic experimental system. It includes a Badal system to change the vergence of the stimulus, a Shack-Hartmann wavefront sensor to measure the refractive state of the eye, a deformable mirror, and a microdisplay showing the stimulus.
EM alone was rarely used in all journals (0–6%). EM was more used in two cell biology journals (20 and 23% in JCB and JCS), less frequent in CEL and PNAS (10%), and in pharmacology and biochemistry journals (6–7%). Although some authors have pointed out the importance of microscopy in drug discovery and in other fields of pharmacology [19, 21], our data showed that microscopy techniques are not widely used in pharmacology research.
The authors analyze the data a conclude that microscopy techniques are infra utilized in pharmacology. While I believe the topic is interesting, the analyses performed fail to support the claims made by the authors. A big rewrite must be done so the authors clarify their claims and analyses. As it is, it is not written clearly enough to be accessible and new results should be included to strengthen the claims. Therefore, I cannot recommend it for publication.
A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
6. Also, it will be very helpful to organize all the relevant factual numbers in one table: number of journals, number of articles in each one. Also add N=… to the figures or figure captions
Example of accommodation to a stimulus with 6 D of AD. Accommodative response measured is 5 D, lagging 1 D. Using its asymmetrical DOFi of 1.2 D (+1.0 to −0.2) about the stimulus vergence, the visual system is able to see the stimulus without an apparent blur although the accommodative lag is 1.0 D.
On pharmacology journals, OM was used in 45–59% articles, whereas cell biology journals used 75–97% of OM. Curiously, use of OM in articles from JBC (53%) was similar to pharmacology journals. Since PNAS is a general journal that publish articles in many subjects that are outside the scope of biological sciences, we analyzed both the percentage of OM use in all articles (32%) of the journal, as well as the percentage of OM use only in articles from the Biological Sciences section of PNAS (47%), which was within the range of OM use in the pharmacology field. It is important to point out that the Biological Sciences section of PNAS included a broad range of fields, such as Agricultural Sciences, Anthropology, Ecology, Evolution, Population Biology, Psychological and Cognitive Sciences, Sustainability Science, and Systems Biology, which only in rare occasions analyze cells and tissues and therefore do not demand the use of microscopy.
Jul 3, 2023 — The eyepiece or ocular lens is the part of the microscope closest to your eye when you bend over to look at a specimen. An eyepiece usually ...
1 - PNAS was one of the journals that was evaluated. Note however, that PNAS is a general journal that publish articles in many subjects that are outside the scope of cell biology, or even biology. Consider how to take this into account (i.e. when quoting in page 8 the percentage of OM, the value is lower, 32%, but I believe that if this will be normalized by the percentage of bio-related publications from all publications, the number will become more similar to that of the cell-bio journals).
Interestingly, live cell microscopy was mostly used by the cell biology journals JCS (42%) and JCB (25%), while the other cell biology journal CEL and all other journals used very rarely (0–6%). A lot of work and some specialized equipment are necessary to use live cell microscopy, but it seems that the results that have being obtained are valued for some cell biology journals.
Reviewer #1: This work studies the use of microscopy in pharmacological research. To do this, the authors propose to do a bibliometrics analysis to review scientific publications during the year 2019 on two pharmacology journals (BJP and FP) and one cell biology journal for control.
- In 211 it is stated that cell biology and pharmacology fields have completely different uses of microscopy techniques”. I do not find clear if the techniques are used for different purposed or it refers that microscopy techniques are more commonly used in cell biology.
1. Some important microscopy methods are not included in the subjects that are tested, including Super resolution microscopy that lately immerged as a very important method.
The position and size of the subject’s pupil were monitored by means of a camera. The pupil center, which is automatically located by the software based on a Hartmann-Shack image, was manually aligned to the simulator’s optical axis. Good accuracy to measure and generate aberrations for the same wavefront sensor and deformable mirror has been reported elsewhere.9,27,28
We also analyzed the use of Cryogenic-electron microscopy (CryEM). Even though CryEM is considered as a revolutionary technique for determining the 3D shape of macromolecules, and therefore has been described as an important technique for drug discovery (Renaud et al. 2018), our analysis revealed that CryEM was only found in articles published in JCB (3%) and PNAS (3.5%), and almost not detected in pharmacology articles. Cryo-EM is most likely being used for basic-science studies, which could explain the low-percentage use.
I would like to state that all listed authors qualify for authorship and agreed in the submission of the manuscript. The final version of the manuscript has been seen and approved by all coauthors. The authors declare that they have no conflict of interest.
If your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information please contact onepress@plos.org.
The percentage of figures containing microscopy images is shown in blue bars, the percentage of articles containing cell culture is shown in orange bars and the percentage of articles containing cell culture and microscopy is shown in magenta. Data were collected from articles published in 2019 in the biomedical sciences journals: British Journal of Pharmacology (BJP), Journal of Pharmacy and Pharmacology (JPP), Frontiers in Pharmacology (FP), Journal of Cell Biology (JCB), Journal of Cell Science (JCS), Cells (CEL), Journal of Biological Chemistry (JBC) and Proceedings of the National Academy of Sciences (PNAS). N = 200 articles analyzed in each scientific journal.
... Wesley Haywood Dean has spent many of his eighty-plus years doing. In this ambitious and inspiring collection of two hundred-plus poems, Dean brings you ...
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/, click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at authorbilling@plos.org.
5 - I may have missed it, but it will be useful to have a table of words usage, the source for the word-cloud images. I t can be a supplement table.
4 - Page 10 lines 222: Cryo-EM is most likely being used for basic-science studies, which explains the low-percentage use.
Author’s response – We now attempted to correlate the use of microscopy with the research theme of each article. Since the themes can be diverse, we focus on analyzing the use of cell cultures, since it is reasonable to expect that microscopes are needed for the proper visualization of cells because of their dimensions. In articles that used cell cultures, we analyzed the percentage of microscopy figures in relation to the total number of figures of the articles. We thank the reviewer for this important suggestion that highly improved our manuscript.
It is not clear where is this fact coming from? Please check the argument, and if necessary, please provide a reference.
FEITO 4 - No specific reasons are given for using such a small sample of journals (3), even if the number of scientific articles is big enough to guarantee that the analysis was not performed by hand.
This section collects any data citations, data availability statements, or supplementary materials included in this article.
We hope that the modifications made in the new version of the manuscript have properly addressed the criticism and suggestions made by the referee, and that the improvements made in the manuscript will be sufficient for its publication in PLOS ONE.
We also analyzed the frequency of words that appear in the title of the articles from the eight selected journals. Wordle™ software (freely available at http://www.wordle.net and created by Jonathan Feinberg) [23] was used to generate a list of words with their relative frequencies, and to generate “word clouds”. The clouds give greater prominence to words that appear more frequently in the source text, i.e., more frequent words appear with larger letters and in a colored gradient. We used the following parameters to generate word clouds: remove common English words and numbers (e.g., “and”, “all”, “to”, “at”), make all words lower-case, Telephoto font type, rounded edges, kindled color, horizontal layout. We only used words that appeared at least three times in all the titles of the 200 articles from each journal. The list of words was manually edited to remove plural words (e.g., “cells” changed to “cell”), different spellings for some words (e.g., “signaling” changed to “signalling”), and symbols (e.g., “NF-kB” changed to “NF-KB”).
2 - Page 8: “Optical microscopy (OM) was much more used than electron microscopy (EM), probably because EM is more labor-intensive than OM”. There are many reasons for the broader use of optical microscopy rather than EM, except for the labor issue. It is also because it is faster, possible to label multiple probes simultaneously, works on live cells, the structure is not damaged during the preparation, it is fully quantitative, and even though the resolution is not as good, it is still good enough to provide sub-cellular information. Most likely, it is more accurate to claim that EM is used only where very high resolution is needed, and OM cannot provide that resolution.
It is a well-known fact that certain optical and neural factors can change the DOFi. One of the most important ones is pupil size1; the presence of chromatic2,3 and monochromatic aberrations4–6 play a significant role as well. The precise value of DOFi also depends on the specific way DOFi is defined. Whereas some definitions are related to an absolute minimum value of visual acuity (VA),2,7 there are others based on relative VA values, for example, dioptric range over which VA exceeds some percentage of its optimum value.8–10 In practice, DOFi is usually based on a simple letter legibility criterion. In this case, the letter size plays an essential role in the final DOFi value.11,12 As a simple example, most presbyopes recognize the visual loss at near distances when trying to read small text, but not with large font sizes, which are nonetheless out-of-focus by the same amount.
FEITO 6 - Finally, the number of references is small and fails to introduce the previous work done in this field. The authors mention the combination of different techniques but fail to cite examples where pharmacology research has improved by the use of microscopy. For example: Usaj MM, Styles EB, Verster AJ, Friesen H, Boone C, Andrews BJ. High-Content Screening for Quantitative Cell Biology. Trends Cell Biol. 2016;26(8):598–611.
The manuscript is now improved according to all the comments and requests by the reviewers. There are still few issues to be refined as listed below.
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact onepress@plos.org.
2. Although the issue of ‘in vivo’ and ‘live cell’ are mentioned in the introduction, these are important issues that should also be part of the study. Otherwise, we are left with partial knowledge, as it is not clear what the usage of the microscopy is.
The techniques analyzed were bright field (BrF), phase contrast (Pha), differential interference contrast (DIC), polarization (Pol), conventional fluorescence (Flu), confocal fluorescence (Conf), super resolution (SRes), and live cell imaging (Live); and 3 types of electron microscopy techniques: transmission (TEM), scanning (SEM) and cryo-EM (CrEM).
Author’s response – We now included data from 8 leading scientific journal from the pharmacology, cell biology, biochemistry, and general biomedical sciences fields. The selected journals were British Journal of Pharmacology (BJP), Journal of Pharmacy and Pharmacology (JPP), Frontiers in Pharmacology (FP), Journal of Cell Biology (JCB), Journal of Cell Science (JCS), Cells (CEL), Journal of Biological Chemistry (JBC) and Proceedings of the National Academy of Sciences (PNAS).




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500