Hex Flange JIS 10.9 - Bolts - jis flange
Capacitive coupling (CC) is non-invasive, thus avoiding these adverse effects. An external power source is required to generate an electrical field between the two parallel conductive layers and the capacitor plates. The cells are evenly stimulated, regardless of their position, via application of a homogenous electrical field without direct contact with the culture medium or the body. However, the need for frequent battery changes often leads to patient noncompliance [101]. Fewer studies have examined capacitive coupling than other methods. Although non-invasive electrostimulation exhibits promise in small animals and in vitro, the electrical field is weaker by the time it reaches the injury site, ultimately resulting in an undesirable outcome [115].
Cell orientation is affected by diverse factors, and electrostimulation can switch the orientation from an alignment that is random to one that is perpendicular or parallel to the electrical field vector, minimizing the cells’ field gradient [84,100]. Cellular arrangement switches from random to directional when the electrostimulation intensity is above 4 V/cm, and this capacity for alignment increases with the electrostimulation intensity [125]. When the duration of electrostimulation is < 2 s, the cell alignment potential is enhanced [126]; however, excessive exposure to electrostimulation, in terms of intensity or duration, can reduce cell viability or lead to cell death via intracellular calcium overload or overproduction of reactive oxygen species (ROS) [125,126]. Electrostimulation enhances the expression of the pro-inflammatory cytokine IL-6 and open voltage-gated ion channels (Cacna1c, Kcn2 and Scn5a) and activates mitogen-activated protein kinase (MAPK) and Rho GTPase signaling [126], potentially improving cell alignment.
During healing, osteoblasts differentiate from heterogeneous sources and MSC types, including endosteal MSCs, periosteal stem cells, angiopericytes, and circulating progenitor cells. Recently, an electrically active biomimetic periosteum was created to improve bone healing by inducing the formation of neurovesicles, which contain neurotrophic factors, thus promoting rBMSC differentiation into osteoblasts [186]. In response to electrostimulation, osteoprogenitor cells increase ECM synthesis, and especially proteoglycan and collagen formation, thereby accelerating endochondral bone formation [187]. By stimulating their electrotaxis, adjuvant electrostimulation improved MSC migration without cytotoxicity, with a corresponding significant increase in the expression of CD73, an MSCs cell-surface marker [187]. Electrostimulation induces cell adhesion and spreading by activating the RhoA/ROCK signaling pathway.
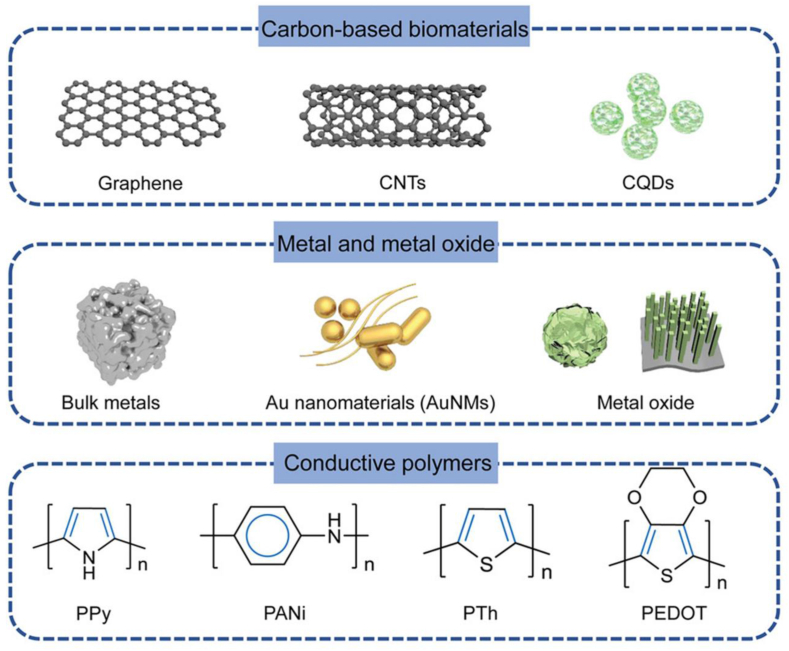
Typical conductive materials in bone regeneration. (Reprinted with permission from Ref. [1]). CNT: carbon nanotube. NP: nanoparticle. PPy: Polypyrrole. PANi: Polyaniline. PTh: Polythiopkene. PEDOT: poly(3,4-ethylenedioxythiophene). CQD: Carbon quantum dots.
The translational potential of this article: This review examines the roles of electroactive biomaterials in rehabilitating the electrical microenvironment to facilitate bone regeneration, addressing current progress in electrical biomaterials and the mechanisms whereby electrical cues mediate bone regeneration. Interactions between osteogenesis-related cells and electroactive biomaterials are summarized, leading to proposals regarding the use of electrical stimulation-based therapies to accelerate bone healing.
Although a patient suffering from a non-union tibial break was successfully treated with electrical stimulation in 1812, the underlying mechanism remained indistinct until Yasuda first elucidated the piezoelectricity of bone [7,8], leading to a new understanding of the associations between electrical stimulation and the piezoelectricity of bone. The increasing research focus on electrical stimulation using different biomaterials is slowly elucidating the underlying mechanisms. Electrostimulation exerts microscopic effects on cell behaviour, such as on cell alignment, adhesion, proliferation, differentiation and migration, as well as macroscopic effects in terms of immune regulation, angiogenesis, osteogenesis, and bone remodelling during bone repair.
Under in vivo electrostimulation, extra ROS are produced in the microenvironment; these include H2O2, organic peroxides, OH radicals, and other Faradaic byproducts, and mitochondrial activity can further increase ROS levels [123]. However, a large study has revealed that, rather than causing cell death due to excessive oxidative stress, intermediate ROS levels can activate MAPK signaling and the subsequent ErK1, ErK2, JNK, and p38 signaling cascades, to some extent improving cell proliferation and differentiation [140,145,146]. This effect is probably associated with the activation of miR-210 [147].
A: The most common chemistries for UV/light curing adhesives are acrylated urethanes. These industrial adhesives have good performance properties, can bond to most materials, and are the least expensive. Cyanoacrylate adhesives are used with tubing and to bond some plastics. Epoxies produce strong bonds but tend to be rigid, which can lead to fracture. Silicones are the most expensive type of adhesive and are available in some formulations that cure with visible light.
Angiogenesis plays an essential role in healing by providing abundant nutrients, oxygen, and growth factors for osteogenesis through the newly formed vascular network [171,172]. Blood vessel formation requires cell proliferation, alignment, elongation, and directed migration of endothelial cells. Notably, electrostimulation enhances angiogenesis and can upregulate angiogenic factors such as VEGF and IL-8 [173,174]. When exposed to direct-current electrical fields of 50–300 mV/mm [175], human mammary epithelial cells (HMECs) mobilized toward the cathode at a velocity positively correlated to the electrical field, whereas this was not observed in the control, indicating that electrostimulation upregulates CXCR4 and CXCR2 expression in HUVECs and HMECs.
A: No. The key is to use a lamp with a spectral output and intensity that matches the requirements of the adhesive. The lamps used for UV/light curing are specified by size or cure area, spectral output or wavelength, and power in W/cm2. Some adhesive formulations are curable with a black light, a relatively inexpensive lamp that emits UV-A; however, the time needed for curing is too long for most production schedules. More powerful lamps can cure adhesives more quickly.
A: Because light energy is required for curing, UV or visible light must reach the bond area. This requires an unobstructed path between the light source and at least one substrate. Part designs that lack fillets or similar geometries may experience “shadowing” and require an adhesive with a secondary moisture cure for complete adhesion. For bonding magnets to metals, an activator that initiates the adhesive reaction may be required. To bond silicone, a UV cure adhesive with a silicone chemistry may be needed.
Electrostimulation can upregulate the transcription of osteogenesis-related genes (Spp2 and Bmp2) and improve phagocytosis and absorption [165,166]. Moreover, M2 macrophages are believed to participate centrally in improving osteoprogenitor-cell osteogenic differentiation, which is affected by the expression of TGF-β, IL-4, BMP-2/4, IL-6, VEGF and other cytokines [167,168]. Indirect modulation of the osseous immunological environment promotes bone repair and accelerates regeneration. Nonetheless, the specific mechanisms and pathways involved remain unclear.
The regenerative capacity of bone is indispensable for growth, given that accidental injury is almost inevitable. Bone regenerative capacity is relevant for the aging population globally and for the repair of large bone defects after osteotomy (e.g., following removal of malignant bone tumours). Among the many therapeutic modalities proposed to bone regeneration, electrical stimulation has attracted significant attention owing to its economic convenience and exceptional curative effects, and various electroactive biomaterials have emerged. This review summarizes the current knowledge and progress regarding electrical stimulation strategies for improving bone repair. Such strategies range from traditional methods of delivering electrical stimulation via electroconductive materials using external power sources to self-powered biomaterials, such as piezoelectric materials and nanogenerators. Electrical stimulation and osteogenesis are related via bone piezoelectricity. This review examines cell behaviour and the potential mechanisms of electrostimulation via electroactive biomaterials in bone healing, aiming to provide new insights regarding the mechanisms of bone regeneration using electroactive biomaterials.
UV Gluefor acrylic
Interested in adding your materials to Gluespec? Just fill out the form below and we will review it and get back with you shortly!
Electrostimulation affects the Coulomb forces in the acellular component of the microenvironment, thus improving bone healing. When an oscillating electric field was used to activate the piezoelectricity of HAβ-PVDF, the negative and positive surface charges were reconfigured to form polar regions attracting Ca2+ and PO4−, respectively [200].
Uv glue and lightfor plastic
Underlying mechanisms of electrical stimulation-induced cell behaviour. 1. Electrostimulation activates G-protein-coupled receptors, and IP3 and DAG are synthesized by phospholipase C (PLC) and subsequently bound to receptors on the endoplasmic reticulum, releasing calcium ions. 2. On the one hand, electrostimulation activates Na+-Ca2+-exchanger (NCX), stretch-activated Ca2+ channels (SACCs), and voltage-gated calcium channels (VGCCs), opening ion channels and causing Ca influx. Ca2+ ions activate calmodulin (CaM)/calcineurin (CaN), promoting cytoskeleton reorganization and NF-AT dephosphorylation which moves into the nucleus to promote the expression of related genes. On the other hand, it activates PKC and MAPK cascades to improve cell proliferation, differentiation, migration, and adhesion. 3. Ca2+ ions activate FAK, leading to the formation of focal contacts and activation of the downstream PI3k/Akt axis to regulate cell behaviour together with other signaling pathways. 4. Electrostimulation affects cell gap junctions, promoting the development and transmission of electroactive cells via exchange of signaling molecules. ECM: extracellular matrix.
CNTs possess distinctive characteristics, making them suitable for bone tissue engineering, including outstanding mechanical robustness and a substantial surface-area-to-volume ratio. Their unique nano-surface structure may create a relatively pro-differentiation and pro-proliferation microenvironment. Carbon-based nanomaterials such as GO can provide a high surface area and abundant functional groups that are suitable for the binding and immobilization of growth factors such as BMP-2 and PDA [81,82]. NPs endow scaffold surfaces with a nanostructure similar to the extracellular matrix (ECM), thereby improving bone-marrow-derived MSC (BMSC) attachment. Positively charged NPs and negatively charged GO nanolayers form a charge-balanced surface on the scaffolds, enhancing BMSC proliferation. In developing these strategies, the focus has been on balancing the mechanical benefits of CNT-based scaffolds and their effects on cell behaviour and viability in the bone microenvironment.
Common carbon-based nanomaterials include fullerenes, graphene, carbon nanotubes (CNTs), and carbon nanofibres, derived from natural carbon allotropes that vary in their covalent bonding to carbon atoms [42,37]. Graphene, a 2D sheet isolated from 3D graphite, presents excellent electrical and thermal conductivity owing to its single-atom-thick structure; a graphene sheet can be curled into a cylinder to form a CNT with a one-dimensional hollow nanostructure and carbon atoms bound to each other via sp2 bonds; this structure thus provides a shared and mobile fourth electron, resulting in electrical conductivity [[37], [38], [77]]. Although the mismatch in mechanical characteristics between bone and carbon-based biomaterials can be modified by incorporating other materials, homogeneous dispersion remains a problem [39]. To overcome this, modifications such as dopamine coating, carboxyl functionalization, amino modification, double-bond functionalization, agent-assisted amphiphilic crosslinking, PEG-modification, and β-cyclodextrin grafting, which improve the surface wettability of CNT and prevent aggregation, are being examined [40].
As Au NPs can perform multiple functions that mediate bone regeneration, the development of Au-based biomaterials has attracted global attention. As with Ag, to make full use of their electroconductivity, Au NPs are loaded into hydrogels or coated onto the scaffold [53]. Au NPs at three concentrations were combined with carbon nanofibres via two different methods [98]. The Au NPs were mixed with an electrospinning solution then simultaneously subjected to electrospinning and electrospraying [98]: the stable CNT/Au NP electroconductive scaffolds produced were reported to have 29.2 % and 81 % higher conductivities, respectively, than CNT-only scaffolds; conductivity was positively related to Au NP concentration, and no cytotoxicity was observed.
Metal/metal oxides, discovered many years ago, are considered as the conventional conductive and exhibit excellent mechanical strength and fatigue resistance. Metal NPs can provide both electrical conductivity and mechanical support. Ag and Au NPs are widely used as surface coatings and dopants in hydrogels. Their high surface free energy facilitates surface modification, and they exhibit a large surface area, porosity, orientation, and excellent conductivity [40]. Ag-based biomaterials are well-studied, FDA-approved, and exhibit extraordinary conductivity [97]; their large surface area enables them to dissolve rapidly, releasing Ag+, which participates in bone healing. Subtoxic concentrations of Ag+ promote MSCs proliferation, migration, and differentiation [49]. A self-promoting electroactive mineralized scaffold was developed to investigate the effects of electrostimulation on the osteogenic differentiation of MSCs and to provide antibacterial potential [50]: Ag was fabricated as ultrathin nanowires and combined with mineralized collagen fibres; in phosphate buffered saline, the carboxyl groups within the collagen accelerated the corrosion of the nanostructured Ag, resulting in a stable current of ca. 4.0 μA. The excellent antibacterial potential of Ag can be attributed to its direct or indirect destruction of cell membranes and walls. However, Ag can also interrupt regular metabolism, leading to ROS generation, which can damage cellular components such as DNA, enzymes, and proteins. Excessive Ag levels may result in dysfunction of osteoblasts and MSCs, undermining rather than promoting healing [51]. Ag cytotoxicity is dose-dependent, and Ag NP size should be considered: 10 nm Ag NPs can jeopardize cell proliferation and differentiation [52].
Piezoelectricity, first discovered by Jacques and Pierre Curie in 1880, is the capacity to transform mechanical force into electricity via a non-centrosymmetric crystal structure [[8], [9], [10]]. Crystals lacking centrosymmetry can also exhibit pyroelectricity, in which polarization is caused by changes in temperature [58]. Ferroelectric materials are pyroelectric materials that exhibit spontaneous polarization [59]. In conclusion, all related crystals are classified into 32 crystal classes, 20 exhibiting piezoelectricity and as a subset of pyroelectric materials, ferroelectric materials exhibit both piezoelectricity and pyroelectricity both [30,59,60].
Ca2+, a common intracellular secondary messenger, activates protein kinase C (PKC) to initiate the MAPK signaling pathway [23]. MAPK is in the serine/threonine kinase family; MAPK signaling involves four cascades, namely extracellular signal-related kinases (ERK1/2), Jun amino-terminal kinases (JNK1/2/3), p38-MAPK, and ERK5. Once activated, these cascades mediate a variety of important cell behaviours and regulate specific mRNA transcription in response to exogenous electrostimulation [140,142]. ERK and p38 activation can induce the expression of RUNX2 and OSX, promoting osteogenic differentiation [23]. Focal adhesion kinase (FAK) is thought to be activated by electrostimulation, and the recruitment of FAK by cytoskeletal anchor proteins can induce binding between phosphotyrosine and Src, activating ERK/MAPK signaling [23,140]. Intracellular Ca2+ accumulation then activates FAK, leading to the formation of focal contacts, and Rho GTPase mediates cytoskeletal organization, together increasing MSCs migration [143].
In a bone defect rat model with a rotary jet-spun implant, inflammatory infiltration was significantly lower following 30 d of external electrostimulation than in the control [169]. Although the underlying mechanisms remain unclear, this is thought to be related to the effect of electrostimulation on mast cells [170].
Both stromal cell-derived factor 1 (SDF-1, the cytokine ligand for CXCR4), and IL-8 (the ligand for the CXCR2) mediate angiogenesis by recruiting endothelial progenitor cells [176,177]. IL-8 improves endothelial cell proliferation and permeability and attracts lymphocytes, macrophages, and neutrophils to perivascular regions [178]. Neutrophils recruited by IL-8 are N2-polarized, subsequently inducing SDF‐1 secretion to recruit BMSCs via the SDF‐1/CXCR4 axis and activating its downstream PI3K/AKT pathway as well as β‐catenin‐mediated migration, creating a positive feedback loop [179]. VEGF improves SDF-1/CXCR4 production and binds to VEGFR, activating PI3K/AKT and Rho/ROCK signaling, thus promoting directional organization of the cytoskeleton and other cell behaviours, and cell migration in particular [179]. The effects of the substratum coating and passage number on HUVEC electrotaxis were eliminated, supporting the influence of electrostimulation [179]. The chemokine receptors CXCR2 and CXCR4 both participate in EC migration, and electrostimulation significantly alters their expression in HUVECs and HMECs. Under electrostimulation, in HMECS, CXCR4 expression peaked at 15 min and CXCR2 expression peaked at 1 h; in HUVECs, CXCR4 expression peaked at 1 h and CXCR2 expression peaked at 30 min. In vitro, electrostimulation significantly enhanced endothelium-derived nitric oxide (NO) levels by upregulating the PI3K/Akt-dependent pathway and VEGF expression [180], which are essential role in angiogenesis. Under electrostimulation, endothelial NOS (eNOS) was phosphorylated and eNOS upregulated, especially in endothelial cells [180], suggesting that electrostimulation-mediated angiogenesis can be largely attributed to the VEGF/VEGFR/PI3K/Akt-eNOS axis (Fig. 3). Electrostimulation stimulates HUVEC proliferation and enhances the S-phase cell population [180]. In a study of CD31, an endothelial cell marker capillary formation, electrostimulation was confirmed to promote angiogenesis [181]. Similarly, electrostimulation enhanced eNOS expression and the level of NO and enhanced fibroblast proliferation without cytotoxic effects [182]. Low-amplitude electricity-enhanced MAPK/ERK signaling stimulated angiogenic vessel-tube formation, and electrostimulation induced fibroblast secretion of fibroblast growth factor 2 (FGF2), a potent angiogenic factor that increases endothelial cell proliferation, improves vessel repair, and protects HUVECs; electrostimulation-induced FGF2 expression thus provides a bridge to initiate angiogenic signaling [182].
Gluespec makes no warranty or guarantee that the information on our Website, product data sheets, product descriptions, prices or other content is current, accurate, complete, reliable, suitable for a particular purpose or error-free. Consult the relevant manufacturer's website and product data sheet for more information.
An external power source is often required to deliver electrostimulation via electroconductive materials such as carbon-based nanomaterials, conductive polymers, and metal/metal oxides. Implantation can cause side effects, and poor patient compliance makes it difficult to conduct large clinical trials. The safety of the CNTs, PANi, and other conductive materials requires further validation to ensure their applicability in clinical trials.
Adding PPy can alter scaffold surface chemistry and roughness, affecting protein deposition from the medium onto the scaffold, thus affecting cell differentiation. Thick, porous scaffolds comprising PEDOT: PSS, gelatin, and bioactive glass NPs enhance the osteogenic differentiation, adhesion, and cell viability of hMSCs, presumably owing to improvements in microstructure and electrical signaling among cells [96]. An electroactive scaffold comprising PPy, alginate, and chitosan improved apatite-layer formation and induced cells to exhibit lamellipodia and filopodia without electrostimulation, potentially owing to the Ca/P ratio of the apatite layer, which is similar to that of natural HA [84].
A: Glass is one of the most popular substrate materials for UV/light cure adhesives and can form high-strength, load-bearing bonds between glass-and-glass, glass-and-plastic, or glass-and-metal. Optical plastics can be joined as well, but materials with low surface energy may produce joints with insufficient strength. With Teflon, for example, a surface treatment such as chemical etching can increase surface energy and contribute to the formation of non-structural bonds.
The addition of functional or peptide-based drugs can govern cell fate and increase bioactivity [38]. Therefore, as alternatives to graphene, graphene oxide (GO) and reduced graphene oxide (rGO), which can be improved by adding oxygen-containing groups such as–OH, –CH(O)CH-, and –COOH [78], are becoming prevalent. However, covalent functionalization may reduce electrical conductivity and cause cytotoxicity, as it could alter sp2 hybridization to tetrahedral sp3 hybridization [79]. A PGO-PHA-AG scaffold exhibited excellent immunomodulatory effects, thus improving osteogenesis; this can be attributed to the introduction of PGO, which increased its conductivity [80]. This scaffold effectively transferred electrical cues to cells, activating Ca2+ channels via electroconductivity. However, various modifications of carbon-based nanomaterials have increased cytotoxicity, particularly following polymer degradation, and the exposure dose remains to the clarified [41]. Further research is therefore required.
A: Microwave-powered, or excited, UV curing lamps have a longer life than traditional metal halide lamps but are more expensive. They are available in commonly used wavelengths and feature an electrodeless bulb that has a long lifetime and applies less heat to the substrate. Microwave-powered UV lamps with integrated blowers are sometimes used in laboratories or other facilities where space is limited.
A: Lamps need to be installed at the proper distance and, depending on the application, may need to provide either flood or focused light. For example, if an adhesive is dispensed onto a printed circuit board (PCB) to bond a small electronic component, a high-intensity spot lamp may be a better choice than a UV/visible conveyor or flood lamp.
In electrostimulation, wave shape influences the cellular response (Table 4). Square-wave electrostimulation increases only M1-associated TLR4 receptor activity [164], because the electrostimulation alters the transmembrane voltage, causing cell depolarization, activating ion channels, and eventually increasing intracellular Ca2+ concentration, thus boosting the TLR signaling-induced activation of NF-κB and ultimately promoting endotoxin/Interferon-γ-induced cascades. In contrast, in BMDMs, sine-wave electrostimulation promotes polarization toward both the M1 and M2 phenotypes (and particularly the M2 phenotype), significantly increasing M1 TLR4 and M2 IL-4Rα activity via gradual changes in the electric field, leading to membrane receptor redistribution [164]. Electrostimulation simultaneously upregulates the M2 cell-membrane receptor IL-4Rα and M1-associated TLR4. IL-4Rα activates STAT6, which perpetuates the IL-4-induced cascade, eventually promoting M2 polarization.
UV Glue and lightKit
Self-powered electroactive biomaterials are beneficial as no external power source is needed. Piezoelectric scaffolds are mostly non-degradable and remain in the body unless surgically removed. Implantable energy harvesters for generating electricity, such as PENGs and TENGs, appear to overcome these limitations. However, these newly developed technologies are far from mature, exhibiting insufficient miniaturization and stabilization.
Owing to differences in cellular responses to electrostimulation, which depend particularly on differences in the composition of the in vitro media, it is difficult to determine the optimal electrical parameters. For osteoblast-like Saos-2 cells, cytosolic Ca2+ levels were substantially elevated under electrostimulation, with the amplitudes of Ca2+ transients compatible with Ca-influx; this was attributed to the opening of VGCCs and SACCs by electrostimulation [159]. In contrast, low-amplitude Ca2+ transients from the endoplasmic reticulum are activated via plasma membrane protein reorganization by PLC and by the opening of lns3P-receptor channels. Cytosolic Ca2+ can trigger the CaM/CaN pathway, resulting in NF-AT dephosphorylation [121]. NF-AT translocates to the nucleus where it binds with other transcription factors to facilitate osteogenic gene expression, especially of TGF-β and BMP. TGF-β participates in activating MAPK and SMAD, thus promoting osteoblast proliferation and differentiation by initiating the Wnt/β-Catenin pathway. TGF-β also enhances MSCs differentiation and migration by activating R-Smads and binding with Smad4 for translocation into the nucleus, where the complex promotes downstream gene expression and regulates ECM production. BMP2, which is upregulated via electrostimulation, binds to the transmembrane tetrameric receptor, thus activating Smad1/5/8 to form a complex with Smad4, which translocates into the nucleus, facilitating RUNX2 transcription [193]. Similarly, the expression of BMP6, in the TGF-β superfamily, was highest expression in the early stage, after which PI3K-Akt signaling was activated, mediating BMSC differentiation [194]. An increase in Ca2+ concentration induces an increase in p38 phosphorylation, mediating osterix expression and thus promoting BMSCs osteogenic differentiation (Fig. 4) [193]. Inhibition of Smad6 was downregulated, contributing to osteogenesis.
During the late stage of inflammation, the highly activated immune response at the injury is suppressed, preventing the healing process from being slowed by long-term inflammation; electrostimulation enhances this by downregulating immune cells and cytokines as well as macrophage polarization [160]. Under electrostimulation, M2 macrophage marker (CD206+ and F4/80+) levels were significantly higher than in the IL-4, IL4+egenerated silk fibroin (RSF), and IL4+0.4 % MXene/RSF groups, whereas this difference was absent when electrostimulation was applied [62]. This indicates that electrostimulation enhanced M1 to M2 macrophage polarization as well as macrophage infiltration potential. Electrostimulation upregulates Cdc42, Rac1, and ROCK expression downstream of the RhoA/ROCK signaling pathway, mediating macrophage polarization, while inhibiting HIF-α protein synthesis and TNF signaling [80]. Electrostimulation significantly inhibits MAPK/JNK cascades, while upregulating ATP synthesis and oxidative phosphorylation [25]. An intact tricarboxylic acid (TCA) cycle, which maintains the energy production via oxidative phosphorylation, is thought to be pivotal to M2 macrophage polarization.
UV Light gluefor glass
Cell adhesion, whereby individual cells form three-dimensional tissues rather than simply sticking together, depends primarily on multiprotein complexes [127]. For successful tissue engineering, grafts or seeded cells require good cell adhesion to attach to scaffolds or implants. Direct current stimulation at 5–25 μA enhanced osteoblast attachment on Ti surfaces in vitro [122]. Human umbilical vein endothelial cell (HUVEC) attachment to a conductive biomaterial surface was greatly enhanced by electrostimulation, especially at 400 mV/cm [128]. PC12 cells exhibited improved adhesion under electrostimulation [129]. Electrostimulation increases production of vinculin, which forms focal sites and recruits other proteins to form adhesive plaques, and upregulates the genes encoding FAK, integrin, NCAM, and N-cadherin; while levels of FAK and integrin, which mediate cell-ECM interactions, were positively correlated, those of NCAM and N-cadherin, which respond to cell–cell conjunction, were negatively correlated [122,130].
A: Sometimes, manufacturers use lamps that emit the wrong type of light for the specific adhesive formulation. Manufacturing processes can also leave inadequate cure time. If a UV/light cure adhesive is stored improperly, such as in a clear bottle that receives sunlight, premature curing may occur. Like other materials, UV/light cure adhesives also have a shelf life beyond which a product becomes unfit for use. If an adhesive fails to stick, however, it’s often because it was the wrong choice for the substate.
Need help? Our knowledgeable experts help you achieve your goals by giving you application insights. They are available personally to help you before, during or after your search.
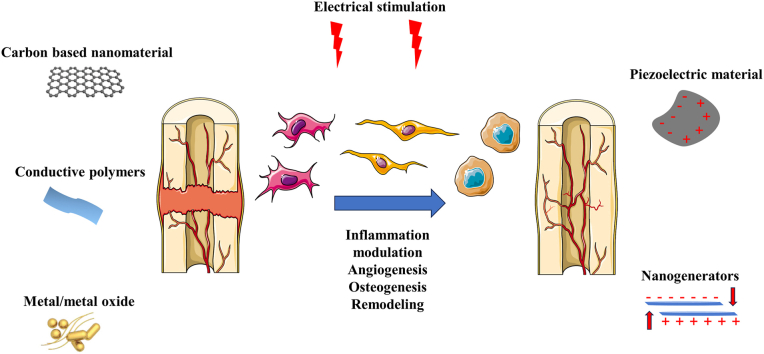
The incidence of musculoskeletal disorders has increased substantially in recent years, presenting a significant challenge in medicine [4]. The application of biophysical stimulation sources such as electricity, ultrasound, and magnetic fields contributes greatly to accelerating bone healing, without the drawbacks associated with conventional treatments, such as the risk of infection and the limited availability of treatments for overly large bone defects [5,6]. Electroactive biomaterials can mimic the bioelectrical properties of natural bone to promote bone regeneration. The conductivity of reconstructed microenvironments has been enhanced by the development of advanced electroactive biomaterials, making it easier to deliver electrical stimulation. Self-powered scaffolds involving piezoelectricity, electrochemical reactions or electrostatic interactions exhibit the advantages of being wireless and electrodeless, providing a favourable solution for promoting bone regeneration. Nonetheless, traditional methods of delivering electrical stimulation are still widely applied both in the scientific field and in clinics, owing to their accessibility and controllability.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Although electrostimulation in bone tissue engineering exhibits potential as a replacement for autogenous bone grafts, its clinical practical application is currently unfeasible. Several preclinical studies using electroactive biomaterials have achieved great progress in improving bone regeneration; nonetheless, there is currently no standard protocol to determine the optimal electrical parameters. The safety of electroactive biomaterial scaffolds requires further exploration to obtain approval by the FDA.
Few studies have investigated the effects of electrostimulation on osteoclasts. Electrostimulation for 28 d reduced osteoclast differentiation and downregulated the expression of Car2, Ctsk, and Acp5, which encode osteoclast enzymes, and of RANK, DC-STAMP, and CD44, indicating reduced osteoclast differentiation. Hereby, in the long term, there was less bone resorption [201]. In vitro, electrostimulation altered culture medium pH and activated osteoblasts and osteoclasts [202]. Nonetheless, the specific mechanisms remain to be elucidated.
Please test and evaluate materials carefully to ensure compliance with all specifications and requirements of your application.
Mitophagy is potential mechanism for the effects of electrostimulation on osteogenic differentiation in rBMSCs [196]. Under electrostimulation, mitochondrial membrane depolarisation was higher than in the control, increasing its potential. LC3, a biomarker of autophagosome formation, was upregulated, reflecting initiation of the Pink I/Parkin pathway, which mediates mitochondrial autophagocytosis; this was followed by lysosomal digestion and finally the release of calcium phosphate to the extracellular matrix to promote bone mineralization.
A: Selecting UV/light cure adhesives requires a complete analysis of factors that include substrate materials, part design, surface energy, wavelength, and lamp selection and installation. Typically, these industrial adhesives are supplied in black syringes, black bottles, or opaque pails. When evaluating suppliers, it helps to choose one who can help with product selection and provide technical assistance. Price, availability, and minimum order quantities (MOQs) are also important considerations.
While electrostimulation is traditionally delivered using exogenous electrical power, many biomaterials exhibit self-powering properties, attracting attention because of their excellent performance in tissue engineering. Innovative and highly conductive materials have been investigated to increase the efficiency of bone regeneration via electrostimulation (Table 1).
Au particles interact with cell membrane receptors, affecting osteogenic differentiation of osteoblast progenitors [54]. Au exhibits great antioxidant potential and enhances the ability of the bound biomolecules to correct the oxidative stress-induced imbalance between osteoblasts and osteoclasts [99]. The promoting effect of Au NPs is size-dependent, with smaller-sized and spherical particles being more effective. During photocatalysis, Au NPs exhibit antibacterial properties by generating ROS [40]. Despite their differences, both Au and Ag show excellent performance in tissue engineering. However, their value as precious metals makes it difficult to realize low-cost manufacturing and substantially reduces their availability for regular medical implantation in clinical practice. Zn and Cu have therefore increasingly been studied as conductive biomaterials.
Organic materials derived from nature have attracted significant attention owing to their excellent biocompatibility, negligible cytotoxicity, and biodegradability. The piezoelectricity of HA and collagen has been widely applied in clinical practice. As piezoelectric materials, silk fibroins from Bombyx mori cocoons, silk from spiders, chitosan extracted via chitin deacetylation, and chitin obtained from crustacean exoskeletons and mushroom cell walls promote bone repair [62,[72], [73], [74]].
Osseous tissue is typically capable of converting external compression into electrical signals [20], creating SGPs. Osseous tissue undergoes corresponding cellular changes in the stressed area to activate and regulate cell signalling related to osteogenesis, osteointegration, and remodelling [16], simultaneously modulating the production of coagulation factors by vascular endothelial cells in the inner layer [8]. Healing is closely related to the electrophysiology of the bone itself; consequently, bone can be considered a true cybernetic self-organizing system [21]. This theory provides the foundation for the application of electrostimulation in bone regeneration, and provides perspectives for exploring the promotion of bone fracture healing, bone defect repair, and osteogenesis by exploiting materials with bioelectrical activities [22].
When deformation occurs, the electrostatic charge in the flowing fluid in the bone attaches to the vessel wall with the opposite electrostatic charge, forming an electric double layer (EDL). Under the influence of the EDL, the flowing liquid exhibits polarity, resulting in a streaming current (Is) and a conducting current (Ic). The stable fall in potential that occurs when the streaming current and conducting current equalise is defined as streaming potential (SP), expressed as follows [14]:
Corresponding author. No. 117 Nanjing North Street, Liaoning Provincial Key Laboratory of Oral Diseases, School and Hospital of Stomatology, China Medical University, Shenyang 110001, China. dentist_ye@163.com
TENGs operate on the principle of the electrical potential difference. When two dissimilar materials move in relation to one another, the material with the higher electrical potential tends to lose electrons, while the other acquires a positive charge, generating an electrostatic force. Movement occurs in vertical contact-separation mode, lateral-sliding mode, single-electrode mode, or freestanding mode [119]. Daily activities can enhance electrical reactions. Recently, self-powered implantable and bioresorbable devices comprising PLGA and Mg have been developed [120]. The vertical contact separation between the bottom PLGA and the top Mg triboelectric layers enables electrons to move between them, and the voltage is correlated with the length of the Mg island, peaking at 4.5 V at lengths of 800–1000 μm. The intensified electrostimulation that this generates enhances bone regeneration, as validated in vivo and in vitro.
UV GluePen
Electrostimulation improves preosteoblast and osteoblast differentiation and osteogenic potential [190,107]. Electrostimulation voltage-dependently and significantly increased the expression of osteogenic markers and genes, particularly of ALP, collagen type I mRNA, and the C-terminal propeptide of collagen type I, relative to the control. Electrostimulation induced the mRNA transcription of COL1A1, RUNX2, SPARC, BGLAP, and SPP1, indicating that it facilitates pre-osteoblast maturation, osteoblast proliferation, and bone matrix formation [[107], [190], [191]].
ROS play a vital immunomodulation, interacting with myeloid-derived suppressor cells to suppress excessive immune reactions [148]. ROS can increase Ca2+ concentration by opening Ca2+ channels [145], and Ca2+ can promote ROS production via CaM [149]. Phosphorylated NF-AT can be dephosphorylated by CaN and delivered into the nucleus, together with transcription factors initiating gene transcription [30,150,151], improving TGF-β and BMP-2 expression and thus regulating cell metabolism and ECM synthesis [30,151].
A: UV/light cure adhesives are designed to cure at specific wavelengths that are measured in nanometers (nm). Visible light has colored bands that range from violet (380-435 nm) to red (625-740 nm). UV light is divided into UV-A (315-400 nm), UV-B (280-315 nm), and UV-C (180-280 nm) but covers a region of the electromagnetic spectrum that is as low as 10 nm. Visible light curing adhesives typically cure from 385nm to 415nm, depending on the formulation. Today, many UV cure adhesives use 365 nm light.
PLA and PLLA, for which biodegradability has been widely validated, have been approved by the U.S. Food and Drug Administration (FDA) as safe for use in implants [68,34]. The piezoelectricity of PLA and PLLA relies both on their crystallinity and molecular orientation, and the electrical dipoles of the C Created by potrace 1.16, written by Peter Selinger 2001-2019 O bands branching out of the polymer backbone are attributed to piezoelectricity. Various processing methods can augment the piezoelectric properties of PLLA. Nano-confined PLLA nanowires, exhibiting 70 % greater crystallinity than unmodified PLLA, switched from α to β phase when the draw ratio reached 2.5 to 4.5, allowing the dipoles to align uniaxially and achieving the best piezoelectric performance [34].
Uv glue and lighthome depot
The development of 2D nanomaterials, including those in the transition metal carbide and carbon nitride (MXene) families, is flourishing owing to their advantages in terms of metal conductivity, high aspect ratio, solution processability, and wide tunability. Black phosphorus (BP), a promising 2D nanomaterial candidate, exhibits outstanding electroconductivity and photothermal effects [62,78]. However, the in vivo stability of 2D nanomaterials requires further improvement owing to their rapid degradation. In a major and exciting breakthrough [83], BP nanosheets were fabricated by integrating silk fibroin (SF) to enhance corrosion resistance; this BP@SF material can easily be processed into different forms such as fibre, film and sponge that can be adjusted to the shape of irregular bone defects. BP has unique antibacterial and antitumor properties that extend its range of applications.
where V is the piezoelectric potential; dijk is a 3-order tensor; L is bone thickness; ε is the dielectric constant; B is the load; σ is conductivity, and t is time.
This review comprehensively summarizes the current research progress on the mechanisms whereby electrostimulation promotes bone regeneration and repair. Common metals such as Ti, Co, and stainless steel, which are biologically inert, are used in bone tissue engineering because of their mechanical properties rather than electrical conductivity, and were therefore excluded from this review. This work aims to provide support and direction for the future exploration of electroactive biomaterial design and to elucidate the mechanisms whereby electrical stimulation drives bone healing, providing a theoretical basis for the application of electrostimulation in orthopaedics, and proposing optimization strategies.
Official websites use .gov A .gov website belongs to an official government organization in the United States.
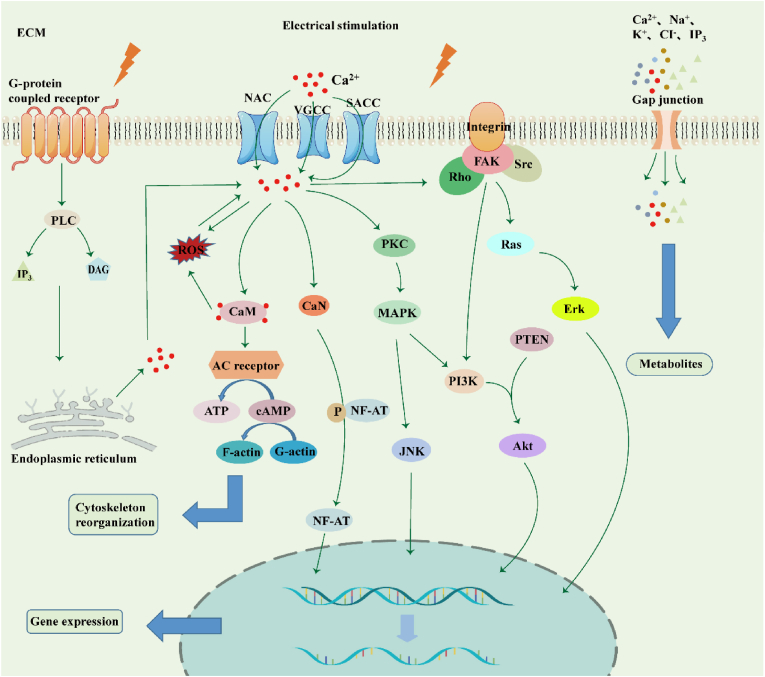
The mechanisms whereby electrostimulation regulates cellular patterns have been widely examined. Electrostimulation plays a significant role in four respects (Fig. 2).
Cell migration, a vital physiological phenomenon that affects various biophysical cues, enables cells to mobilize to certain areas to perform functions that enhance healing, immunomodulatory activity, and other physiological activities. Cells from multiple tissue types display directed migration under electrostimulation, a phenomenon known as electrotaxis; for instance, neural stem cells, macrophages, mouse neural precursor cells, osteoblasts, and endothelial progenitor cells mobilize toward the cathode, while BMSCs, human dermal fibroblasts, and SCs move toward the anode [84]. The direction of cell movement is consistent with Golgi apparatus polarization in cells under electrostimulation [135], suggesting that electrostimulation might affect cell migration by causing Golgi apparatus polarization. For ADSCs, the effects of a single period of electrostimulation lasted for ca. 6 h, while continuous electrostimulation did not accelerate the time required for cell migration [135]. Using a bioelectric healing-on-a-chip platform, it was found that electrostimulation could drive collective cell migration and promote wound healing and closure [136,137]. Such collective migration is presumably related to high calcium concentrations and junctional E-cadherin [138].
Mechanisms of electrostimulation affecting osteogenic cells. Electrostimulation activates voltage-gated calcium channels (VGCCs) and stretch-activated Ca2+ channels (SACCs), causing Ca2+ influx, triggering Ca2+ release by activating PLC and Ins3P receptor channels on the endoplasmic reticulum; this causes NF-AT dephosphorylation via the CaM/CaN pathway and thus promotes the expression of osteogenic genes, especially TGF-β and BMP. When BMP binds to cells, the key transcription factor TGF-β participates in activating MAPK and SMAD, promoting osteoblast proliferation and differentiation by initiating the Wnt/β-Catenin pathway, and enhancing differentiation and migration by regulating extracellular matrix (ECM) production and osteogenic gene expression. Under electrostimulation, DNMT1 is immediately downregulated, while OCT4 and NANOG gene expression is significantly upregulated via demethylation of their promoters, thus maintaining their osteo-differentiation-inducing ability.
Electrostimulation for bone regeneration is currently a hot topic. Electroactive implants that rehabilitate the electrical microenvironment, either by generating electricity or using external power sources, have been have successfully developed. The use of highly effective implants has led to rapid regeneration of injured tissue. The mechanisms whereby electrical cues drive bone healing have been elucidated. From conventional wires and electrodes to advanced devices that transform another type of energy produced during daily activity into electricity, there has been considerable progress in the exploration of electroactive biomaterials.
Insulating materials are those that do not allow the free movement of electrons and therefore lack electrical conduction. However, a continuum of highly polarizable materials exists; when an electrical field is applied to such materials, tightly bound positive and negative charges become aligned in certain directions, and an additional electrical field is observed [30]. Various types of polarization have been postulated to explain the generation of electricity via polarization, including ionic polarization (Pi), electronic polarization (Pe), dipolar polarization (Pd) and interfacial polarization (Pint) [55,56]. Polarization disrupts the balance between the positive and negative charges within a material by distorting the dipole moment of each affected molecule [57]. The polarizability of materials is measured in terms of the dielectric constant [30]. Polarization is related to physical stimuli including mechanical stress and changes in temperature, or can occur spontaneously.
For direct coupling (DC) in vitro, conductive electrodes are placed inside the cell-culture wells in contact with the culture medium and cells. This in vitro approach is typically considered invasive, as it may result in infection, bleeding, or even non-union. The production of reactive Faradic byproducts from electrochemical reactions (hydrogen peroxide, hydroxyl ions, and other free radicals) [100], changes in pH, or the oxidation of bare metallic electrodes can liberate traces into the cell culture medium. Cells closest to the electrodes can undergo morphological changes. Despite these disadvantages, direct coupling is widely used, particularly for in vitro studies, because of its availability.
This review examines the roles of electroactive biomaterials in rehabilitating the electrical microenvironment to facilitate bone regeneration, addressing current progress in electrical biomaterials and the mechanisms whereby electrical cues mediate bone regeneration. Interactions between osteogenesis-related cells and electroactive biomaterials are summarized, leading to proposals regarding the use of electrical stimulation-based therapies to accelerate bone healing.
In inductive coupling (IC), a pulsed electromagnetic field (PEMF) of low-level electromagnetic signals is generated using a conductive coil or solenoid. An alternating current at the fracture site flows perpendicularly to this magnetic field, mimicking the physiological process in the body. This approach avoids the formation of undesirable byproducts. However, the optimal parameters, such as the magnetic field density, frequency, pulse duration, and stimulation time, remain under debate. The intrinsic link between the electrical and magnetic fields in the PEMF makes it difficult to distinguish these effects separately. Fortunately, transformer-like coupling-based devices can apply electrostimulation without interference from the magnetic field [116,117], thus revealing the effects of electrostimulation.
A newly designed CFO/P(VDF-TrFE) membrane with an electrical potential gradient was applied to investigate the effects of electrostimulation on rBMSCs: the potential gradient(Δζ) mediated the optimal osteogenic capacity was calculated to be 0.672 pm/(V × μm). Levels of the integrins α5β1 and αvβ3 may be upregulated, facilitating cytoskeleton rearrangement and their tight adhesion to membrane proteins [188]. In MSCs, PEMF electrostimulation at 7.5 Hz upregulated the expression of ALP and osteocalcin, especially when BMP-2 was bound to the MSCs; electrostimulation activated RUNX2/CBFA1, a pivotal transcription factor, promoting their osteogenic potential [189].
Corresponding author. No. 115 Nanjing North Street, Department of Plastic Surgery, The First Hospital of China Medical University, Shenyang, 110001. China. sguo@cmu.edu.cn
The polymer biomaterial polymethyl methacrylate (PMMA) bone-cement was infiltrated into BT scaffolds to form a lamellar composite structure with a layered piezoelectric phase aligned in the same direction, achieving a higher piezoelectric coefficient of 6–39 pC/N. The compressive strength of BaTiO3/PMMA composite, which exhibits a structure mimicking that of natural shell, increased from 39 to 111 MPa with an increase in BT content [65]; similarly, its Young's modulus (E) increased with BT content, which was close to that of bone tissue. In addition to PMMA, polycaprolactone (PCL) was also under consideration to produce a PCL/BaTiO3 composite scaffold, exhibiting porosity of 35 %–45 %, within the range of that of human cancellous bones; the addition of BT particles (at 10 wt%) significantly improved its mechanical strength (to 54 ± 0.5 MPa) and d33, facilitating electricity generation [26].
Electrostimulation can alter the electrical field of ECM proteins, altering cell adhesion and growth; this is especially pronounced when it is combined with biomaterial implantation, in which protein attachment to the biomaterial is accelerated [197,198]. Electrostimulation improved MSCs differentiation potential by epigenetically regulating DNA modification and gene expression [199]: DNMT1 was immediately downregulated, whereas OCT4 and NANOG gene expression was significantly upregulated, with the demethylation of their promoters, maintaining the subsequent osteogenic differentiation of MSCs. For DNMT1 expression to regulate OCT4 and NANOG expression, the electrostimulation must not exceed 10 ns at 20 kV/cm or 100 ns at 10 kV/cm.
Ferroelectricity and pyroelectricity occur in bone tissue [15]. However, current theory holds that strain-generated potentials in bone (SGPs) are the sum of the piezoelectric potential and SP [16]. Under normal physiological conditions, human bone is subjected to mechanical forces in various directions owing to daily activities such as walking and standing; minimal negative charges are generated in the area under compression, whereas positive charges are generated in the stretched area with respect to other areas [17]. The voltage in the human tibia is about 300 μV during walking [18]. Bone repair strictly follows Wolff's Law [19], which indicates that: 1) the osteoblasts are relatively active on the stress axis; 2) that newly regenerated bone tissue typically comprises robust lamellar bone rather than tissue outside of the stress axis, where osteoclasts are relatively active and superfluous callus is gradually absorbed and decayed.
Potassium sodium niobate (KNN), barium titanate (BT), and other lead-free ceramics compared to lead zirconate titanate (PZT), which can release toxic elements and cause accumulation of hazardous electronic waste [33,61]. BT exhibits an asymmetric structure only below the Curie temperature (120 °C), below which free movement of Ti4+ and O2− prevent it from. The stress caused the dipole change functions as temperature to commence phase transformation. BT therefore possesses both piezoelectricity and ferroelectricity; while it can polarize spontaneously in the human body, it can also generate electrical cues in response to mechanical force, thus exhibiting great potential in promoting bone regeneration. Although the biocompatibility of BT has been verified [59,[23], [24], [25]], its application is limited by its poor processability, weak mechanical strength, and fragility when used for large bone defects, especially those with irregular shapes [26]. Multiple modifications have been applied to address these problems. To provide stable mechanical support, a 3D-printed BT-coated scaffold was developed; this exhibited piezoelectricity, the robust mechanical properties of Ti6Al4V, and improved wettability [62]. However, simply coating the scaffold with BT failed to mimic the natural microenvironment and tomography of bone tissue [62]. Thus, hydroxyapatite (HA), a natural component of bone tissue, was applied in combination with BT to create a porous HA/BaTiO3 piezoelectric composite, which exhibited a compressive strength close to that of cancellous bone (2–12 MPa). As its porosity increased from 40 % to 60 %, its compressive strength declined from 42.6 MPa to 17.5 MPa, and the piezoelectric coefficient (d33) of 50 % porous HA/BaTiO3 composite reached 5.0 pC/N [63]. As the proportion of HA increased, the d33 of the composite decreased: when the proportion of BT in the composite was <70 %, its piezoelectricity was hardly detectable despite the significant cell growth observed, and the composite exhibited ferroelectricity only when its BT content exceeded 95 % [64].
Bone fracture healing, a complex and dynamic process of transition from an anabolic to a catabolic phase, comprises three consecutive and partially overlapping phases: the early inflammatory phase, repair, and remodelling [152]. These phases involve inflammation, cell recruitment (proliferation and migration), vascularization, osteogenic differentiation, and remodelling, and the secretion of abundant biomolecules [153]. Electrostimulation at these different stages has key relevance as an adjuvant therapy with various effects, improving bone healing performance.
We have no control over the conditions of use of materials, so (even if you use the Ask an ESR feature) it is solely up to you to independently determine the efficacy, suitability, performance and safety of the material for your application.
Like BT, polyvinylidene fluoride (PVDF) crystalizing in β-phase exhibits both piezoelectricity and ferroelectricity [68]. In contrast, its other phases (α, γ, δ, and ε) lack a net dipole moment and are thus nonpiezoelectric. However, α-PVDF can transform into β-PVDF via mechanical stretching; this alters its conformation from trans-gauche to all-trans, creates an electrical pole to stabilize the dipole, and eventually results in piezoelectricity [30]. PVDF, which exhibits high flexibility and stiffness, can be fabricated precisely using several methods, such as fiber electrospinning, 3D printing, selective laser sintering, and rapid prototyping [30,42]. 3D PVDF-based scaffolds exhibit an effective biomimetic microenvironment with excellent potential for promoting cell adhesion and proliferation [69]. Electrospun poly[vinylidenefluoride-co-trifluoroethylene] (PVDF-TrFE) fibrous scaffolds exhibit better piezoelectricity and electromechanical coupling coefficients than PVDF, owing to the greater presence of β-phase PVDF; PVDF-TrFE is therefore gradually being considered the better alternative [68,70]. The elastic modulus of PVDF-TrFE is similar to that of cancellous bone, avoiding stress shielding, particularly when used in weight-bearing areas. PVDF-TrFE combined with BT NPs achieved better human mesenchymal stem cell (hMSC) viability and promotion of osteogenesis than when used separately [31]. Nonetheless, some limitations exist in the clinical utilization of PFVDF, as it exhibits strong resistance to degradation, requiring surgery to remove the material, or eliciting an inflammatory response, considering that the PVDF implant becomes integrated into the newly formed bone [32]. Long-term PVDF implantation of may cause heart failure or release ultratoxic acid molecules [71]. Therefore, the synthesis of biodegradable piezoelectric polymers, such as polylactic acid (PLA) and poly l-lactic acid (PLLA), a PLA conformation containing L-stereoisomers [33], has been investigated.
ZnO, which exhibits three different crystal forms, exhibits piezoelectricity when present in wurtzite [27]. Zn, a trace element in humans, plays an essential role in many biological activities, including immunomodulation, arterial blood pressure regulation, and bone regeneration, directly mediating bone formation and mineralization via aminoacyl-tRNA synthetase and inhibiting osteoclast-like differentiation [28]. More importantly, ZnO is biodegradable and shows an intermediate corrosion rate relative to Mg- and Fe-based materials. ZnO nanoparticles (NPs) can generate electricity, causing various cellular behaviours and anti-bacterial and anti-tumour effects, while also generating ROS owing to their electron-transport ability; dose-dependent ROS-induced cytotoxicity can thus arise from the presence of Zn in biomaterials [29]. Under normal conditions, based on its mechanical properties and relative fragility, Zn is not appropriate for use with bone tissue. Therefore, various methods to combine Zn with other elements have been developed to improve its strength and ductility and thus avoid premature implant failure.
Uv glue and lightnear me
PANi, a conductive polymer with good electrical conductivity and mechanical stability, can be synthesized using low-cost aniline. PANi can be classified based on its oxidation level, among which polyaniline emeraldine (which is half-oxidized) exhibiting the best stability and electroconductivity [93]. Polyaniline eliminates ROS and exhibits adequate anti-bacterial potential, making it an attractive choice for promoting bone regeneration. However, PANi is nondegradable and has been reported to cause notable chronic inflammation in vivo [44]. Low-molecular-weight oligoanilines were recently reported to possess similar conductivity to their high-molecular-weight analogues; they accelerate macrophage phagocytosis and could be used to reduce chronic inflammatory responses, averting secondary surgery [90,45]. Although PANi is insoluble in common solvents and is hydrophobic, chemical grafting and in situ polymerization can significantly improve its degradability; the number of electrons associated with electrical conductivity in the main chain remains constant as long as the PANi is not involved in redox doping [46,47].
Pulsed electrical field electrostimulation (>5 V/mm) upregulated the expression of the growth factors VEGF, BDNF, and NGF, activating p38 signal transduction [144]. The complex signaling network that synergistically mediates cell regulation includes the Wnt signaling pathway for osteogenesis, PI3K/PTEN signaling, and its downstream protein Ark which participates in cell survival and apoptosis.
A: Strength is especially important. Manufacturers list this information on an adhesive’s data sheet in terms of pounds per square inch (psi) or its metric equivalent. Shear strength, a property that describes an adhesive’s resistance against a shear load before failure, is typically measured with an Instron. Speed of cure and depth of cure are also critical because engineers need adhesives that cure quickly but completely. Viscosity is application-dependent and determines whether an adhesive spreads easily or remains in a bead. Typically, depth of cure and viscosity are more important for pottants and encapsulants than for bonding or conformal coatings.
All authors have read and approved the article. Concept and design: S.Y.L, Q.W. and C.S.Z. Drafting of the manuscript: S.Y.L., C.S.Z., Y.P.S.. Revision of the manuscript for important intellectual content: H.Z.Z. W.X. Q.W., H.Y.L., S.G. and S.D.Y. Supervision: S.G., H.Y.L. and S.D.Y.
PEDOT provides a better alternative to PPy than PANi, because it exhibits higher oxidation resistance and better conductivity. Unlike PPy, PEDOT maintains higher conductivity under the same circumstances, exhibiting no cytotoxicity and successfully improving cell growth, adhesion, and differentiation [94,48]. Combining PEDOT with the polyelectrolyte polystyrene sulfonate (PSS), forming PEDOT: PSS, dramatically increased the conductivity of the polymer system and exhibited water solubility, facilitating film formation; this increased conductivity is due to the weak Coulomb forces between the PEDOT and PSS chains [78]. Although other dopants, such as sulfated alginate and chitosan, can similarly improve the dispersion of PEDOT, PSS remains the most effective dopant [40]. A hydrogel was formed by dispersing PEDOT:PSS, CaP, and MgSiO3 evenly at specific proportions into methacrylated alginate to form a hydrogel [95]; with the introduction of PEDOT:PSS, the hydrogel exhibited notable redox peaks and its conductivity increased to ca. 1.52 ± 0.09 mS/cm. Significant bone regeneration was observed when an alternating voltage of 0.5 V at 100 Hz was applied [95].
Electroactive biomaterials enhance bone regeneration via their effects on immunomodulation, angiogenesis, osteogenesis, and bone remodelling. Combining electrostimulation and bone tissue engineering achieves long-lasting benefits with minimal side effects. The remaining obstacles to address include insufficient biodegradability, stability, processability, and undesirable mechanical properties. Bone regeneration is complex and dynamic, involving a wide range of cell types. The optimal electrical parameters and duration for therapy therefore require further investigation.
In bone tissue engineering, polarized KNN combined with bioactive glass shows promise for accelerating angiogenesis; however, its electrical properties are greatly influenced by temperature [33,66]. Moreover, Li-modified KNN (LKNN) shows outstanding chemical stability and hydrophilicity toward interstitial fluids, implying better biocompatibility than KNN. In contrast, LKNN ceramics exhibit excellent electrical characteristics (d33 = 222), and polarized LKNN bolsters osteoblast growth and may be a favorable implant material for use in orthopaedics [67].
Developments in materials science and composite design have made self-powered electrostimulation an attractive option for the modulation and repair of bone defects. The application of electrically conductive materials somewhat reduces energy loss and increases bioactivity. Self-powered electrical technologies can be classified into four categories: triboelectric nanogenerators (TENGs), piezoelectric nanogenerators (PENGs), magnetoelastic generators (MEGs) and biofuel cells [118]. Biofuel cells are rarely used to promote bone regeneration. MEGs involve a magnetic force, which is beneficial during bone healing. Therefore, in this review, we focus on TENGs and PENGs.
The effects of various electrostimulation methods on bone healing in vivo and in vitro have been tested. In vitro, electrostimulation is applied to cells through tissue culture plates using special bioelectrical or conductive scaffolds. Three primary coupling methods are used: direct, capacitive, and inductive [23,100,101]. Table 2, Table 3 summarize the specific parameters and related mechanisms [91,95,98,[102], [103], [104], [105], [106]].
A: Lamps that use light emitting diodes (LEDs) have longer-lasting bulbs, use less energy, and produce less heat. They also create strong bonds because their light output can be tightly focused on the required wavelength for optimum cure. Unlike metal halide lamps, however, LED lamps cannot provide the broad performance that may be needed if manufacturers decide to use various UV/light cure adhesives with different curing requirements.
Bestuv glue and light
This work was supported by the China Medical University High Quality Development Program (No.2022JH2/20200064), National Natural Science Foundation of China (82301045), the Liaoning Medical-Engineering Joint Fund (2022-YGJC-16).
Electrostimulation enhances cell proliferation and adhesion, the first steps in osteogenesis. In addition to activating cellular pathways, electrostimulation influences ECM proteins to promote cell proliferation and attachment. Proteins diffuse to the site and are subsequently absorbed onto the surface to facilitate cell activity [192]. Differences in electrical field frequency, voltage, duration of application, and other electrical parameters can cause gene expression to be triggered at different time points and alter the peak rates of IL-6, OPN, OPG, and DKK-1 expression; nonetheless, the underlying mechanisms and specific responses remain to be revealed.
PENGs produce piezoelectricity as a result of their unique crystalline structure. Stress deforms the structure, resulting in electrical charge accumulation. Piezoelectric materials have been widely used in self-powered implants to investigate the effects of electrostimulation on osteogenesis. Piezoelectric polyhydroxybutyrate@zinc oxide (PHB@ZnO) nanofibres and chitosan were used to fabricate a 3D nanofiber-aerogel scaffold, using ultrasonography to simulate pressure: the scaffold exhibited superior piezoelectric generation at a ZnO level of 2 wt%, with a maximum output voltage and current of 800 mV and 0.4 μA at a PHB@ZnO nanofibre/chitosan mass ratio of 6:4 [121]. The electrical cues generated by the scaffold enhanced rBMSC differentiation and significantly promoted mineralization; this presumably involves electrostimulation-induced Ca2+ influx and subsequent activation of calmodulin (CaM)/calcineurin (CaN)/NF-AT signaling [121].
Under local electrical fields, sensory nerves, which are abundant in the periosteum, are activated to secrete the neuropeptides VIP, SST, and CGRP, inducing rBMSCs to express more osteogenic-related genes via the CGRP/FAK/VEGF and Wnt signaling pathways [186]. Electrostimulation is known to exert its effects by altering Ca2+ levels. Under electrostimulation, ROS production is enhanced via interactions with Ca2+, increasing over time with electrostimulation, leading to a pathological process [123]. By introducing a TiO2/Bi2O3 heterojunction with a surface potential of ca. 110 mV onto a nanoscale interface, built-in electrical fields were applied, significantly enhancing e osteogenic gene expression via the PI3K signaling pathway [195].
Similarly, macro-piezoelectricity can be generated using electrospun aligned nanofibers. HA, Mg, and other materials are combined with PLLA to govern scaffold degradation, providing robust mechanical support before healing and avoiding the side-effects caused by long-term implantation; these modified PLLA scaffolds are assumed to take ca. 5.7 years to degrade completely in the human body [35,36]. Appropriately processed PLLA is widely used in designing piezoelectric nanogenerators to exploit the shear-piezoelectric mode, to achieve optimal piezoelectricity. Although the mechanical properties of PLLA are relatively poor compared to those of PVDF, and it generates relatively unstable and weak electrostimulation, it is adequate for improving osteogenesis [33,42,34].
A: Unlike heat cure adhesives, UV/light cure adhesives do not require ovens for curing, the chemical process by which an adhesive attains its final properties, including strength. Instead, lamps that produce specific wavelengths of light are used. UV/light cure adhesives are also free of solvents and available in one-part formulations that do not require time-consuming mixing. Some UV-curing adhesives are formulated with a secondary moisture-curing mechanism for “shadowed” areas that aren’t reachable by the UV lamp. In this sense, they have some similarities with moisture-cure adhesives.
Bone tissue inherently possesses piezoelectricity—the capacity to accumulate electrical charge—resulting in polarization in response to small deformations caused by mechanical stress [8]. The piezoelectric effect is believed to arise mainly form collagen fibres in the bone, as this fibre exhibits a non-centrosymmetric crystal structure [9]. Further, collagen molecules are rich in –CO– and –NH– groups, which can be considered dipoles. Under mechanical stress, these dipoles are rearranged as the collagen fibres slide past each other, and the dipoles become oriented along the long axis of the bone. Consequently, the centres of the positive and negative charges are separated to produce a piezoelectric effect [10]. However, based on mathematical studies, hydroxyapatite, that exhibits a hexagonal crystal system, lacks an inversion center and crystallizes in the centrosymmetric space, causing it to exhibit piezoelectricity; this has been confirmed via piezoresponse force microscopy [11]. Bone tissue piezoelectricity is negatively correlated with humidity [12], especially when the humidity reaches 40 % [13], bone piezoelectric potential is difficult to measure. Hydration results in the formation of hydrogen bonds when H2O binds to collagen molecules, thus increasing its molecular structural symmetry, thus reducing the polarized charge. However, hydroxyapatite can prevent this binding, making it easier for the collagen fibres to exhibit a piezoelectric effect. Thus, the piezoelectric potential under physiological conditions, such as in fluid-saturated bone, can be expressed as follows [13]:
The inflammatory response begins immediately after bone injury, when the blood vessels supplying the bone tissue and periosteum rupture, causing hematoma at the fracture area. The clotting hematoma serves as a temporary framework for recruiting inflammatory cells and various cytokines for subsequent bone regeneration. Pro-inflammatory cytokines such as TNF-α, BMP, IL-1, IL-6, IL-11 and IL-23 are secreted at the injury, initiating the inflammatory cascade and then recruiting macrophages. Monocytes, lymphocytes and other immune cells collectively modulate the immunological microenvironment in fracture area [154], making the microenvironment conducive to bone healing and regeneration. Early research revealed that the electrical current that is immediately generated by nerves at the wound creates a suitable electrical environment, facilitating changes in cellular DNA, RNA and proteins [155]. This current is indispensable for the bone regeneration process, with the electrostimulation promoting blastema formation, which initiates healing; exogenous electrostimulation accelerates the commencement of the process [21,155]. Early in inflammation, faster recruitment of immune cells and cytokines enhances bone regeneration; therefore, adjuvant electrostimulation to rapidly recruit macrophages, monocytes, and lymphocytes to the injury can accelerate the repair [[156], [157], [158]]; this acceleration is assumed to be related to the opening of voltage-gated K+ channels and ERK phosphorylation. Electrostimulation tends to enhance macrophage motility, tending to cause long-distance displacement of macrophages [159].
While the underlying mechanisms of cellular responses to electrostimulation remain obscure, hyperpolarization of the cell membrane potential is known to activate the Na+-Ca2+-exchanger (NCX), stretch-activated Ca2+ channels (SACCs), and voltage-gated calcium channels (VGCCs) on the cell membrane [30,139,140]. Ca2+-coupling signaling pathways begin to activate, eventually causing a Ca2+ influx [30,123,139]. Partial electrostimulation can alter the structure of transmembrane receptors (such as factor receptors, integrin-β molecules, and adenosine A2A receptors) by inducing asymmetric assembly and disassembly of F-actin filaments, thus initiating related signaling pathways via ligand-receptor binding [1]. For instance, G-protein-coupled receptors are activated, allowing phospholipase C (PLC) to synthesize inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), which can bind to receptors on the endoplasmic reticulum and release its stored Ca2+ into the cytoplasm [140]and triggering the expression of calmodulin (CaM) and calcineurin (CaN) [22,139]. Subsequently, cytoskeletal CaM is activated to further promote cell proliferation and growth factor expression; adenylyl receptors are then activated to synthesize more ATP, which can be further consumed for the conversion of monomeric G-actin to polymeric F-actin, achieving reorganization of the actin cytoskeleton [1]. Via its effects on cell gap junctions, which can exchange electrical and metabolic coupling, electrostimulation promotes the development of electroactive cells, as well as cellular communication, via the exchange of signaling molecules such as Ca, K, cyclic nucleotides, and inositol phosphates [141].
For mouse pre-osteoblasts (MC3T3 cells), direct-current electrostimulation at 100–1000 mV/mm significantly enhanced cell growth, with the amplitude being increased over an 11 d period [109]. The transcription factors RUNX2 and OSTX were expressed, further suggesting that electrostimulation can induce guided osteogenic differentiation [109]; when electrostimulation intensified to 500 mV/mm, ALP activity, cell maturation, and differentiation potential peaked [109]. Similarly, in human adipose-derived MSCs (ADSCs), hMSCs and mouse BMSCs, electrostimulation enhances the expression of ALP, RUNX2 and other factors, improving their osteogenic differentiation [23,130,131]. In rat pheochromocytoma cells (PC12 cells), relative to the control, electrostimulation for 72 or 120 h increased the number of PC12 cells by 4.3 and 4.5 times, while voltages of 60, 120 and 240 mV increased it by 5.6, 6, and 6.8 times, respectively, indicating that cell proliferation increases with the intensity of the electrostimulation [132]. Fibroblasts exhibited similar responses under electrostimulation [133]. In addition to promoting osteogenic differentiation, in vivo and in vitro, mRNA- and protein-level analysis revealed that electrostimulation caused by Au nanostrips caused neural stem cells to differentiate into neurons [134].
Adhesive dispensing can be done automatically but is often done manually instead. For higher production volumes, a robotic dispenser can provide a relatively quick return on investment (ROI). Even a simple desktop robot offers advantages in terms of speed, consistency, and reliability. For spot dispensing, a robot equipped with a light wand can be used for curing – if the light is shielded from the dispense tip, to prevent curing in the tip.
The conjugated molecular backbone of polymers results in innate rigidity and brittleness. Although combining them with other materials can improve their mechanical properties, making them soluble and easy to process, the gradual degradation of the other supporting materials poses a safety concern [87,88]. The electrical conductivity of PPy declines with long-term electrical circulation, particularly via de-doping, and the experiment of the PPy coated PLA scaffold approved [89].
Website Content, Database Data and Arrangement of Data and Related Software Copyright 2014-2024 Ellsworth Adhesives. All rights Reserved.
Electrostimulation induces angiogenesis during bone regeneration. Electrostimulation promotes the secretion of SDF-1, IL-8, MCP-1, FGF2, and VEGF. Activation of the SDF-1/CXCR4 axis promotes the initiation of PI3K/AKT and β-Catenin, which upregulate eNOS levels and increase intracellular NO, promoting cell proliferation. On the other hand, it also mediates cell migration via β-Catenin. VEGF and VEGFR together activate Rho/Rock signaling to promote cytoskeletal remodelling, and activate the PI3K/Akt-eNOs axis. Electrical stimulation increases CXCR2, CXCR4, and CD31 expression on the cell membrane surface, further promoting endothelial cell migration.
where V is the streaming potential; ξ is the zeta potential; ε is the dielectric constant; ΔP is the voltage difference between the ends of the tube; σ is the conductivity; and η is the viscosity coefficient.
Corresponding author. No. 115 Nanjing North Street, Department of Plastic Surgery, The First Hospital of China Medical University, Shenyang, 110001. China. sdyang@cmu.edu.cn
To minimize energy consumption and improve biocompatibility, electroconductive biomaterials have been developed as biomimetic platforms to promote tissue regeneration [75]. There are three types of electroconductive biomaterials: carbon-based nanomaterials, conductive polymers, and metal/metal oxides (Fig. 1) [42,76]. Recently, carbon-based nanomaterials and conductive polymers have been investigated [76].
A: Metal halide lamps use more energy, but some UV/light cure adhesives need them in order to cure quickly and with all of an adhesive’s end-use properties. Plus, a standard 365 nm metal halide lamp covers a broad spectrum ranging from visible light to UV-B. Yet, metal halide lamps have a shorter life than LED lamps. Metal halide curing lamps also degrade with first use and may have power issues even when they light-up. This can contribute to inadequate curing.
PPy can be loaded with bioactive molecules owing to its large specific surface area and suitability for surface modification. A heparin-doped PPy/PLA membrane was fabricated to investigate the effect of the composite on promoting transdifferentiation, achieving remarkable results. Proteins and polysaccharides are popular choices for incorporation into PPy [90]. The conductivity of PPy-coated PLLA fibres was better than that of a simple PLLA scaffold. And the significant osteogenic differentiation was illustrated, especially when exerting electrical stimulation in 2 groups. It's well demonstrated that electrical property exaltation can be beneficial for bone regeneration [91]. Considering that PPy responds to electrical signals, electrically controlled drug-delivery systems are possible [92].
Polypyrrole (PPy), polyaniline (PANi), and poly(3,4-ethylenedioxythiophene) (PEDOT) are the most common conductive polymers used in tissue engineering [42,76]. Their electroconductivity is mostly due to their alternate single and double bonds and to the fact that the electrons loosely held by π-conjugated bonds can move freely, creating an electrical path. Polymers are usually easily synthesized, and their biocompatibility has been widely validated [84,43]. PPy exhibits excellent electroconductivity. Polymers are easy to process and modify [37]. More importantly, PPy particles can be applied separately or in combination with other metals; this is most often achieved by incorporating them into hydrogels and electrospun scaffolds [85]. Doping PPy with anions such as Clˉ, Brˉ, or NO3ˉ alters the shape and position of the current peaks as well as its physical morphology. However, as the release of ions can be hazardous, the dopants should be chosen with caution [86].
Industrial adhesives that cure with visible or ultraviolet (UV) light form fast, strong chemical bonds between substrates. Visible light curing adhesives are becoming increasingly popular, and demand for UV cure adhesives is projected to grow steadily. For design engineers who are considering these products, this Q&A from GlueSpec® provides answers to frequently asked questions (FAQs).
A: UV/light cure adhesives typically do not come in colors, although some are available in black. Clarity counts because color absorbs light and can affect curing. Consequently, most UV cure adhesives are clear, translucent, or opaque. For glass or plastic surfaces where the coating or bonding layer should not be visible, such as in optical applications, choose an adhesive with the same refractive index as the substrate material itself. Not all data sheets list optical specifications, however.
Appropriate electrostimulation can activate diverse signaling pathways and influence the intracellular microenvironment [100]. Under electrostimulation, the membrane potential is hyperpolarized [68], significantly affecting the alignment of various cell types [84] as well as cell adhesion [122,123], proliferation, differentiation [109], and migration [124], and significantly promoting other cell behaviours.
A: The main advantages are reduced energy consumption, faster assembly times, low levels of volatile organic compounds (VOCs) for fewer environmental health and safety (EH&S) concerns, and the ability to form flexible, stress-absorbing bonds between similar or dissimilar substrates. UV/light cure adhesives also allow for the proper alignment of components before bonding, which typically occurs in seconds. In addition, it’s easy to scale-up production by adding more lamps to an assembly line.
Bioelectricity underlies the functions of electrically active organs and tissues [1]. In the human body, endogenous bioelectrical signals carried by ions and electrons mediate the regulation of cell-, tissue-, and organ-level patterning and behaviour [2,3]. From the accidental discovery of membrane potential to the exploration of excitation conductivity, bioelectricity plays an important role in physiological and pathological processes ranging from cell alignment, adhesion, proliferation, differentiation and migration to tissue regeneration [1]. Increasing attempts are being made to mimic the natural electrophysiological microenvironment of cells and tissues, particularly in tissue engineering, to facilitate the repair process.
The effects of electrostimulation on macrophage polarization depend on various parameters. Electrostimulation via a pulse capacitive coupling electrical field (PCCEF) with pulse width ≥10 μs enhanced macrophage polarization toward the M1 phenotype and YPA/TAZ expression, thus enhancing IL-6 expression [161]. The built-in electrical field in the micro-area periodically activated VGCCs, promoting Ca2+ influx and initiating the PI3K/Akt/mTOR signaling pathway, facilitating macrophage polarization toward the M2 type, and upregulating cytokine expression, thus enhancing tissue repair and creating an immune microenvironment conducive to cell differentiation [161]. Bone marrow-derived macrophage (BMDM) polarization increased with electrostimulation, whereas excessive electrostimulation prevented polarization [162]. The optimum electrostimulation voltage to enhance polarization, triggering cellular responses, is 500 mV [162]. Ultrasound-driven piezoelectric discharge was found to regulate polarization toward the M1 via Ca2+ influx through voltage-gated channels and establishment of the Ca2+/CAMK2A/NF-κB axis, enhancing the pro-inflammatory macrophage response [163].
Appropriate electrostimulation causes significant VEGF and FGF-1 expression around the fracture site, with an increase in new blood vessel formation, indicating that it strongly bridges osteogenesis and angiogenesis [120,183]. Electrostimulation upregulates growth factor secretion, upregulating the expression of ATP metabolism-related genes [183], and especially that of oxidative phosphorylation-related transmembrane complexes I, III, and V. It upregulates the activity of ATP-assisted ion channel activity, important cation channels. The MAPK/ERK signaling pathway increases VEGF-A expression, affecting functional vessel formation [184]. Electrostimulation enhances angiogenesis by strengthening the pro-angiogenic potential of ADSCs: it enhances secretion of paracrine angiogenic factors such as VEGF and MCP-1, while significantly reducing that of the anti-angiogenic factor Serpin E1/PAI-1 [185].




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500