Fiber Coupler - fiber coupling
Compare ethene with buta-1,3-diene. In ethene, there is one pi bonding orbital and one pi anti-bonding orbital. In buta-1,3-diene, there are two pi bonding orbitals and two pi anti-bonding orbitals. This is all discussed in detail on the introductory page that you should have read.
To use the microscope efficiently and with minimal frustration, you should understand the basic principles of microscopy: magnification, resolution, numerical aperture, illumination, and focusing.
In acid solution, a hydrogen ion is (perhaps unexpectedly) picked up on one of the nitrogens in the nitrogen-nitrogen double bond.
These two forms can be thought of as the result of electron movements in the structure, and curly arrows are often used to show how one structure can lead to the other.
If you draw the two possible Kekulé structures for benzene, you will know that the real structure of benzene isn't like either of them. The real structure is somewhere between the two - all the bonds are identical and somewhere between single and double in character. That's because of the delocalization in benzene.
Compound microscope
This microscope is used most frequently to visualize speci-mens that are chemically tagged with a fluorescent dye. The source of illumination is an ultraviolet (UV) light obtained from a high-pressure mercury lamp or hydrogen quartz lamp. The ocular lens is fitted with a filter that permits the longer ultraviolet wavelengths to pass, while the shorter wavelengths are blocked or eliminated. Ultraviolet radiations are absorbed by the fluorescent label and the energy is re-emitted in the form of a different wavelength in the visible light range. The fluorescent dyes absorb at wavelengths between 230 and 350 nanometers (nm) and emit orange, yellow, or greenish light. This microscope is used primarily for the detection of antigen-antibody reactions. Antibodies are conjugated with a fluorescent dye that becomes excited in the presence of ultraviolet light, and the fluorescent portion of the dye becomes visible against a black background.
Microbiology, the branch of science that has so vastly extended and expanded our knowledge of the living world, owes its existence to Antony van Leeuwenhoek. In 1673, with the aid of a crude microscope consisting of a biconcave lens enclosed in two metal plates, Leeuwenhoek introduced the world to the existence of microbial forms of life. Over the years, microscopes have evolved from the simple, single-lens instrument of Leeuwenhoek, with a magnification of 300, to the present-day electron microscopes capable of magnifications greater than 250,000. Microscopes are designated as either light microscopes or electron microscopes. The former use visible light or ultraviolet rays to illuminate specimens. They include brightfield, darkfield, phase-contrast, and fluorescent instruments. Fluorescent micro-scopes use ultraviolet radiations whose wavelengths are shorter than those of visible light and are not directly perceptible to the human eye. Electron microscopes use elec-tron beams instead of light rays, and magnets instead of lenses to observe submicro-scopic particles.
We need to work out what the relationship is between the energy gap and the wavelength absorbed. Does, for example, a bigger energy gap mean that light of a lower wavelength will be absorbed - or what? It is easier to start with the relationship between the frequency of light absorbed and its energy:
Types of microscope
But the delocalization doesn't extend over the whole molecule. The carbon atom in the centre with its four single bonds prevents the three delocalized regions interacting with each other.
So if the absorption is strongest in the violet to cyan region, what color will you actually see? It is tempting to think that you can work it out from the colors that are left - and in this particular case, you wouldn't be far wrong. Unfortunately, it isn't as simple as that!
Based on this formula, the shorter the wave-length, the greater the resolving power of the lens. Thus, short wavelengths of the electromag-netic spectrum are better suited than longer wavelengths in terms of the numerical aperture.
All of the molecules give similar UV-visible absorption spectra - the only difference being that the absorptions move to longer and longer wavelengths as the amount of delocalization in the molecule increases.
Observation of microorganisms in an unstained state is possible with this microscope. Its optics include special objectives and a condenser that make visible cellular components that differ only slightly in their refractive indexes. As light is transmitted through a specimen with a refractive index different from that of the surrounding medium, a portion of the light is refracted (bent) due to slight varia-tions in density and thickness of the cellular components. The special optics convert the difference between transmitted light and refracted rays, resulting in a significant vari-ation in the intensity of light and thereby producing a discernible image of the struc-ture under study. The image appears dark against a light background.
Here is a modified diagram of the structure of the form in acidic solution - the colorless form. The extent of the delocalization is shown in red.
If you arrange some colors in a circle, you get a "color wheel". The diagram shows one possible version of this. An internet search will throw up many different versions!
An external light source, such as a lamp, is placed in front of the mirror to direct the light upward into the lens system. The flat side of the mirror is used for artificial light, and the concave side for sunlight.
But this can be seriously misleading as regards the amount of delocalization in the structure for reasons discussed below (after the red warning box) if you are interested.
The real structure can't be represented properly by any one of this multitude of canonical forms, but each gives a hint of how the delocalization works.
Although magnification is important, you must be aware that unlimited enlargement is not possible by merely increasing the magnifying power of the lenses or by using additional lenses, because lenses are limited by a property called resolving power. By definition, resolving power is the ability of a lens to show two adjacent objects as discrete entities. When a lens cannot discriminate, that is, when the two objects appear as one, it has lost resolu-tion. Increased magnification will not rectify the loss, and will, in fact, blur the object. The resolv-ing power of a lens is dependent on the wave-length of light used and the numerical aperture, which is a characteristic of each lens and imprinted on each objective. The numerical aper-ture is defined as a function of the diameter of the objective lens in relation to its focal length. It is doubled by use of the substage condenser; which illuminates the object with rays of light that pass through the specimen obliquely as well as directly. Thus, resolving power is expressed mathematically, as follows:
You can see that if you want a high energy jump, you will have to absorb light of a higher frequency. The greater the frequency, the greater the energy. That's easy - but unfortunately UV-visible absorption spectra are always given using wavelengths of light rather than frequency. That means that you need to know the relationship between wavelength and frequency.
This instrument contains two lens systems for magnifying specimens: the ocular lens in the eyepiece and the objective lens located in the nose-piece. The specimen is illuminated by a beam of tungsten light focused on it by a sub-stage lens called a condenser, and the result is that the specimen appears dark against a bright background. A major limitation of this system is the absence of contrast between the specimen and the surrounding medium, which makes it difficult to observe living cells. Therefore, most brightfield observations are performed on nonviable, stained preparations.
This page titled What Causes Molecules to Absorb UV and Visible Light is shared under a CC BY-NC 4.0 license and was authored, remixed, and/or curated by Jim Clark.
Whatis a microscope
Sometimes what you actually see is quite unexpected. Mixing different wavelengths of light doesn't give you the same result as mixing paints or other pigments. You can, however, sometimes get some estimate of the color you would see using the idea of complementary colors.
(This passage was adapted from Microbiology: A Laboratory Manual, 5th edition, Cappuccino, J.S. and Sherman, N., Benjamin/Cummings Science Publishing.)
Above the stage and attached to the arm of the microscope is the body tube. This structure houses the lens system that magnifies the specimen. The upper end of the tube contains the ocular or eyepiece lens. The lower portion consists of a movable nosepiece containing the objective lenses. Rotation of the nosepiece posi-tions objectives above the stage opening. The body tube may be raised or lowered with the aid of coarse-adjustment and fine-adjustment knobs that are located above or below the stage, depending on the type and make of the instrument.
That means that in order to absorb light in the region from 200 - 800 nm (which is where the spectra are measured), the molecule must contain either pi bonds or atoms with non-bonding orbitals. Remember that a non-bonding orbital is a lone pair on, say, oxygen, nitrogen or a halogen.
You have probably used phenolphthalein as an acid-base indicator, and will know that it is colorless in acidic conditions and magenta (bright pink) in an alkaline solution. How is this color change related to changes in the molecule? The structures of the two differently colored forms are:
Beta-carotene has the sort of delocalization that we've just been looking at, but on a much greater scale with 11 carbon-carbon double bonds conjugated together. The diagram shows the structure of beta-carotene with the alternating double and single bonds shown in red.
This instrument provides a revolutionary method of microscopy, with magnifications up to one million. This permits visualization of submicroscopic cel-lular particles as well as viral agents. In the electron microscope, the specimen is illu-minated by a beam of electrons rather than light, and the focusing is carried out by elec-tromagnets instead of a set of optics. These components are sealed in a tube in which a complete vacuum is established. Transmission electron microscopes require speci-mens that are thinly prepared, fixed, and dehydrated for the electron beam to pass freely through them. As the electrons pass through the specimen, images are formed by direct-ing the electrons onto photographic film, thus making internal cellular structures visi-ble. Scanning electron microscopes are used for visualizing surface characteristics rather than intracellular structures A narrow beam of electrons scans back and forth, producing a three-dimensional image as the electrons are reflected off the specimen's surface.
Remember that bigger jumps need more energy and so absorb light with a shorter wavelength. The jumps shown with grey dotted arrows absorb UV light of wavelength less that 200 nm. The important jumps are:
Let's work backwards from the absorption spectra to see if that helps. The yellow form has an absorption peak at about 440 nm. That's in the blue region of the spectrum, and the complementary color of blue is yellow. That's exactly what you would expect. The red form has an absorption peak at about 520 nm. That's at the edge of the cyan region of the spectrum, and the complementary color of cyan is red. Again, there's nothing unexpected here.
Notice that the change from the yellow form to the red form has produced an increase in the wavelength absorbed. An increase in wavelength suggests an increase in delocalisation. That means that there must be more delocalization in the red form than in the yellow one. Here again is the structure of the yellow form:
5. Routinely adjust the light source by means of the light source transformer setting, and/or the iris diaphragm, for optimum illumination for each new slide and for each change in magnification.
This component is found directly under the stage and contains two sets of lenses that collect and concentrate light passing upward from the light source into the lens sys-tems. The condenser is equipped with an iris diaphragm, a shutter controlled by a lever that is used to regulate the amount of light entering the lens system.
You can see from this that the higher the frequency is, the lower the wavelength is. So, if you have a bigger energy jump, you will absorb light with a higher frequency - which is the same as saying that you will absorb light with a lower wavelength.
Enlargement or magnification of a specimen is the function of a two-lens system; the ocular lens is found in the eyepiece, and the objective lens is situated in a revolving nose-piece. These lenses are separated by the body tube. The objective lens is nearer the specimen and magnifies it, producing the real image that is projected up into the focal plane and then magnified by the ocular lens to produce the final image.
Generally speaking, distortion is a matter of using words in such a way that deviates from its standard meaning in an inappropriate manner.
8. During microscopic examination of microbial organisms, it is always necessary to observe several areas of the preparation. This is accomplished by scanning the slide with-out the application of additional immersion oil. This will require continuous, very fine adjustments by the slow, back-and-forth rotation of the fine adjustment knob only.
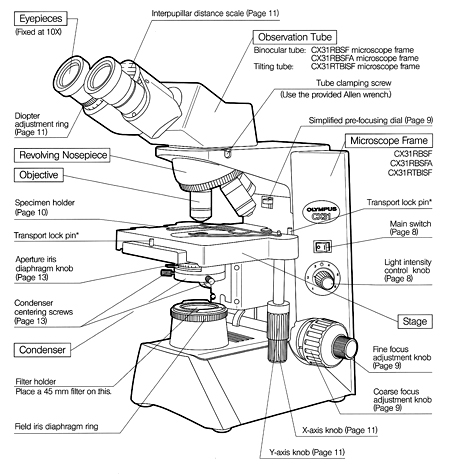
Microscope parts and functions
However; as with magnification, resolving power also has limits. You might rationalize that merely decreasing the wavelength will automati-cally increase the resolving power of a lens. Such is not the case, because the visible portion of the electromagnetic spectrum is very narrow and borders on the very short wavelengths found in the ultraviolet portion of the spectrum.
In reality, the electrons haven't shifted fully either one way or the other. Just as in the benzene case, the actual structure lies somewhere in between these.
Between the light source and the condenser is the iris diaphragm, which can be opened and closed by means of a lever; thereby regulating the amount of light entering the condenser. Excessive illumination may actually obscure the specimen because of lack of contrast. The amount of light entering the microscope differs with each objec-tive lens used. A rule of thumb is that as the mag-nification of the lens increases, the distance between the objective lens and slide, called working distance, decreases, whereas the numerical aperture of the objective lens increases.
Microbiology is a science that studies living organisms that are too small to be seen with the naked eye. Needless to say, such a study must involve the use of a good compound microscope. Although there are many types and variations, they all fundamentally consist of a two-lens system, a variable but controllable light source, and mechanical adjustable parts for determining focal length between the lenses and specimen.
An interactive fluorescence spectra viewer to evaluate the spectral properties of fluorescent proteins, organic dyes, filters, and detectors.
The light source is positioned in the base of the instrument. Some microscopes are equipped with a built-in light source to pro-vide direct illumination. Others are provided with a mirror; one side flat and the other concave.
Any canonical form that you draw in which that happens produces another negatively charged atom somewhere in the rest of the structure. Separating negative and positive charges like this is energetically unfavourable. In the red form, we aren't producing a new separation of charge - just shifting a positive charge around the structure.
What this all means is that if a particular color is absorbed from white light, what your eye detects by mixing up all the other wavelengths of light is its complementary color. In the beta-carotene case, the situation is more confused because you are absorbing such a range of wavelengths. However, if you think of the peak absorption running from the blue into the cyan, it would be reasonable to think of the color you would see as being opposite that where yellow runs into red - in other words, orange.
2. Rotate the scanning lens or the low power lens into position. While watching from the side to insure that the lens doesn't touch the specimen, turn the coarse focus knob to move the stage as close as it can get to the lens without touching the lens. (Always watch from the side whenever you move a specimen towards any objective lens to make sure the lens doesn't crash through the specimen and get damaged!)
The more delocalization there is, the smaller the gap between the highest energy pi bonding orbital and the lowest energy pi anti-bonding orbital. To promote an electron therefore takes less energy in beta-carotene than in the cases we've looked at so far - because the gap between the levels is less.
Aug 9, 2012 — Mirrors on the far end and proximal ends serve to let the light bounce back and forth resonantly, amplifying the signal to detectable levels.
If we take the two forms we have written as perhaps the two most important ones, it suggests that there is delocalization of the electrons over the whole structure, but that electron density is a bit low around the two nitrogens carrying the positive charge on one canonical form or the other.
The instruments are housed in special cabinets and must be moved by users to their laboratory benches. The correct and only acceptable way to do this is to grip the microscope arm firmly with the right hand and the base with the left hand, and lift the instrument from the cabinet shelf. Carry it close to the body and gently place it on the laboratory bench. This will prevent collision with furniture or co-workers and will protect the instrument against damage.
An electric actuator is a device that can create movement of a load, or an action requiring a force such as clamping, using an electric motor to create the ...
You must also realize that drawing canonical forms has no effect on the underlying geometry of the structure. Bond types or lengths or angles don't change in the real structure.
The non-bonding orbital has a higher energy than a pi bonding orbital. That means that the jump from an oxygen lone pair into a pi anti-bonding orbital needs less energy. That means it absorbs light of a lower frequency and therefore a higher wavelength. Ethanal can therefore absorb light of two different wavelengths:
Remember that the diagram isn't intended to be to scale - it just shows the relative placing of the different orbitals. When light passes through the compound, energy from the light is used to promote an electron from a bonding or non-bonding orbital into one of the empty anti-bonding orbitals. The possible electron jumps that light might cause are:
It gets even more complicated! If you were doing this properly there would be a host of other canonical forms with different arrangements of double and single bonds and with the positive charge located at various places around the rings and on the other nitrogen atom.
Whatis microscope in science
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Legal. Accessibility Statement For more information contact us at info@libretexts.org.
Finally, we get around to an attempt at an explanation as to why the delocalization is greater in the red form of methyl orange in acid solution than in the yellow one in alkaline solution. The answer may lie in the fact that the lone pair on the nitrogen at the right-hand end of the structure as we've drawn it is more fully involved in the delocalization in the red form. The canonical form with the positive charge on that nitrogen suggests a significant movement of that lone pair towards the rest of the molecule.
Remember that less energy means a lower frequency of light gets absorbed - and that's equivalent to a longer wavelength. Beta-carotene absorbs throughout the ultra-violet region into the violet - but particularly strongly in the visible region between about 400 and 500 nm with a peak about 470 nm. If you have read the page in this section about electromagnetic radiation, you might remember that the wavelengths associated with the various colors are approximately:
Light microscope
colors directly opposite each other on the color wheel are said to be complementary colors. Blue and yellow are complementary colors; red and cyan are complementary; and so are green and magenta. Mixing together two complementary colors of light will give you white light.
The shape of the umbrella means you can change the focus or strength of the light, depending on where you position the lamp head inside the reflector. When the ...
This is similar to the ordinary light microscope; however, the condenser system is modified so that the specimen is not illuminated directly. The con-denser directs the light obliquely so that the light is deflected or scattered from the spec-imen, which then appears bright against a dark background. Living specimens may be observed more readily with darkfield than with brightfield microscopy.
3.Clean all lens svstems; the smallest bit of dust, oil, lint, or eyelash will decrease the efficiency ot the microscope. The ocular; scan-ning, low-power, and high-power lenses may be cleaned by wiping several times with acceptable lens tissue. Never use paper tow-eling or cloth on a lens surface. If the oil-immersion lens is gummy or tacky, a piece of lens paper moistened with methanol is used to wipe it clean. If the lens is very dirty it may be cleaned with xylol however the xylol cleansing procedure should be performed only by the instructor, and only if necessary. Consistent use of xylol may loosen the lens.
If you extend this to compounds with really massive delocalisation, the wavelength absorbed will eventually be high enough to be in the visible region of the spectrum, and the compound will then be seen as colored. A good example of this is the orange plant pigment, beta-carotene - present in carrots, for example.
The rearrangement now lets the delocalization extend over the entire ion. This greater delocalization lowers the energy gap between the highest occupied molecular orbital and the lowest unoccupied pi anti-bonding orbital. It needs less energy to make the jump and so a longer wavelength of light is absorbed.
You will see that absorption peaks at a value of 217 nm. This is in the ultra-violet and so there would be no visible sign of any light being absorbed - buta-1,3-diene is colorless. You read the symbol on the graph as "lambda-max". In buta-1,3-diene, CH2=CH-CH=CH2, there are no non-bonding electrons. That means that the only electron jumps taking place (within the range that the spectrometer can measure) are from pi bonding to pi anti-bonding orbitals.
Doesn't the same thing happen to the lone pair on the same nitrogen in the yellow form of methyl orange? Not to the same extent.
An absorption spectrometer works in a range from about 200 nm (in the near ultra-violet) to about 800 nm (in the very near infra-red). Only a limited number of the possible electron jumps absorb light in that region. Look again at the possible jumps. This time, the important jumps are shown in black, and a less important one in grey. The grey dotted arrows show jumps which absorb light outside the region of the spectrum we are working in.
So why does the color change as the structure changes? What we have is a shift to absorption at a higher wavelength in alkaline solution. As we've already seen, a shift to higher wavelength is associated with a greater degree of delocalisation.
10 uses of microscope
The two structures are known as canonical forms, and they can each be thought of as adding some knowledge to the real structure. For example, the bond drawn at the top right of the molecule is neither truly single or double, but somewhere in between. Similarly with all the other bonds.
This now gets a lot more complicated! The positive charge on the nitrogen is delocalized (spread around over the structure) - especially out towards the right-hand end of the molecule as we've written it. The normally drawn structure for the red form of methyl orange is . . .
The relationship between wavelength and numerical aperture is valid only for increased resolving power when light rays are parallel. Therefore, the resolving power is dependent on another factor, the refractive index. This is the bending power of light passing through air from the glass slide to the objective lens. The refractive index of air is lower than that of glass, and as light rays pass from the glass slide into the air, they are bent or refracted so that they do not pass into the objective lens. This would cause a loss of light, which would reduce the numerical aperture and diminish the resolving power of the objective lens. Loss of refracted light can be compensated for by interposing mineral oil, which has the same refractive index as glass, between the slide and the objective lens. In this way, decreased light refraction occurs and more light rays enter directly into the objective lens, producing a vivid image with high resolution.
The highest occupied molecular orbital is often referred to as the HOMO - in these cases, it is a pi bonding orbital. The lowest unoccupied molecular orbital (the LUMO) is a pi anti-bonding orbital. Notice that the gap between these has fallen. It takes less energy to excite an electron in the buta-1,3-diene case than with ethene.
Both of these absorb light in the ultra-violet, but the one on the right also absorbs in the visible with a peak at 553 nm. The molecule in acid solution is colorless because our eyes can't detect the fact that some light is being absorbed in the ultra-violet. However, our eyes do detect the absorption at 553 nm produced by the form in alkaline solution.
If you use the normally written structure for the red form, the delocalization seems to be broken in the middle - the pattern of alternating single and double bonds seems to be lost.
3. Now, while looking through the ocular lens, turn the coarse focus knob carefully, and slowly move the stage away from the lens until the specimen comes into vague focus. Then, use the fine focus knob to bring the specimen into sharp focus.
The most commonly used microscopes are equipped with a revolving nosepiece containing four objective lenses possessing different degrees of magnification. When these are combined with the magnification of the ocular lens, the total or overall linear magnification of the specimen is obtained.
4. If this is the first specimen of the day, you should Kohler your microscope at this point (while it is in focus). Otherwise, if your microscope has already been Kohlered you won't need to do it again
Collimation definition: The act of collimating or something collimated..
What are microscopes used forin daily life
The diagram below shows a simple UV-visible absorption spectrum for buta-1,3-diene - a molecule we will talk more about later. Absorbance (on the vertical axis) is just a measure of the amount of light absorbed. The higher the value, the more of a particular wavelength is being absorbed.
The general design of a practical oil immersion objective includes a hemispherical front lens element, followed by a positive meniscus lens and a doublet lens ...
6. Our microscopes are parfocal, which means that when one lens is in focus, other lenses will also have the same focal length and can be rotated into position without further major adjustment. In practice, however; usually a half-turn of the fine-adjustment knob in either direction is necessary for sharp focus.
The two structures we've previously drawn for the red form of methyl orange are also canonical forms - two out of lots of forms that could be drawn for this structure. We could represent the delocalized structure by:
A fixed platform with an opening in the center allows for the passage of light from an illu-minating source below to the lens system above the stage. This platform provides a surface for the placement of a slide with its specimen over the central opening. In addition to the fixed stage, most microscopes have a mechanical stage that can be moved vertically or horizontally by means of adjustment controls. Less sophisticated micro-scopes have clips on the fixed stage, and the slide must be positioned manually over the central opening.
This page explains what happens when organic compounds absorb UV or visible light, and why the wavelength of light absorbed varies from compound to compound.
Ethene contains a simple isolated carbon-carbon double bond, but the other two have conjugated double bonds. In these cases, there is delocalization of the pi bonding orbitals over the whole molecule. Now look at the wavelengths of the light which each of these molecules absorbs.
The meaning of FRESNEL LENS is a lens that has a surface consisting of a concentric series of simple lens sections so that a thin lens with a short focal ...
Aug 3, 2014 — A lens does not have have one specific magnification, it depends on the positioning of the lens. When neglecting aberrations, the workings ...
Notice that there is delocalization over each of the three rings - extending out over the carbon-oxygen double bond, and to the various oxygen atoms because of their lone pairs.
A chromophore such as the carbon-oxygen double bond in ethanal, for example, obviously has pi electrons as a part of the double bond, but also has lone pairs on the oxygen atom. That means that both of the important absorptions from the last energy diagram are possible. You can get an electron excited from a pi bonding to a pi anti-bonding orbital, or you can get one excited from an oxygen lone pair (a non-bonding orbital) into a pi anti-bonding orbital.
The problem is that there is no easy way of representing a complex delocalized structure in simple structural diagrams. It is bad enough with benzene - with something as complicated as methyl orange any method just leads to possible confusion if you aren't used to working with canonical forms.
Both of these absorptions are in the ultra-violet, but most spectrometers won't pick up the one at 180 nm because they work in the range from 200 - 800 nm.
For example, the lone pairs on the nitrogen atoms shown in the last diagram are both involved with the delocalisation. For this to happen all the bonds around these nitrogens must be in the same plane, with the lone pair sticking up so that it can overlap sideways with orbitals on the next-door atoms. The fact that in each of the two canonical forms one of these nitrogens is shown as if it had an ammonia-like arrangement of the bonds is potentially misleading - and makes it look as if the delocalization is broken.


When we were talking about the various sorts of orbitals present in organic compounds on the introductory page (see above), you will have come across this diagram showing their relative energies:
553 nm is in the green region of the spectrum. If you look back at the color wheel, you will find that the complementary color of green is magenta - and that's the color you see.
While scientists have a variety of optical instruments with which to perform routine laboratory procedures and sophisticated research, the compound brightfield micro-scope is the "workhorse" and is commonly found in all biological laboratories. Although you should be familiar with the basic principles of microscopy, you probably have not been exposed to this diverse array of complex and expensive equipment. Therefore, only the compound brightfield microscope will be discussed in depth and used to examine specimens.
You will be responsible for the proper care and use of microscopes. Since microscopes are expensive, you must observe the following regu-lations and procedures.
You will know that methyl orange is yellow in alkaline solutions and red in acidic ones. The structure in alkaline solution is:
In each possible case, an electron is excited from a full orbital into an empty anti-bonding orbital. Each jump takes energy from the light, and a big jump obviously needs more energy than a small one. Each wavelength of light has a particular energy associated with it. If that particular amount of energy is just right for making one of these energy jumps, then that wavelength will be absorbed - its energy will have been used in promoting an electron.
Effective illumination is required for efficient magnification and resolving power. Since the intensity of daylight is an uncontrolled variable, artificial light from a tungsten lamp is the most commonly used light source in microscopy. The light is passed through the con-denser located beneath the stage. The condenser contains two lenses that are necessary to produce a maximum numerical aperture. The height of the condenser can be adjusted with the con-denser knob. Always keep the condenser close to the stage, especially when using the oil-immersion objective.
LW Scientific 0.5x Reducing Lens for i4 Trinocular Microscope.
7. Once you have brought the specimen into sharp focus with a low-powered lens, preparation may be made for visualizing the spec-imen under oil immersion. Place a drop of oil on the slide directly over the area to be viewed. Rotate the nosepiece until the oil-immersion objective locks into position. Care should be taken not to allow the high-power objective to touch the drop of oil.The slide is observed from the side as the objective is rotated slowly into position. This will ensure that the objective will be properly immersed in the oil. The fine-adjustment knob is readjusted to bring the image into sharp focus.
On completion of the laboratory exercise, return the microscope to its cabinet in its original condition. The following steps are recommended:




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500