Boats Should Be Sleek—But Only Up to a Point - length and beam
Types ofobjectivelenses
Chemical reactions that do not depend on the concentration of reactants are called zero-order reactions. Example: Radical reaction of alkanes to haloalkanes in the presence of light.
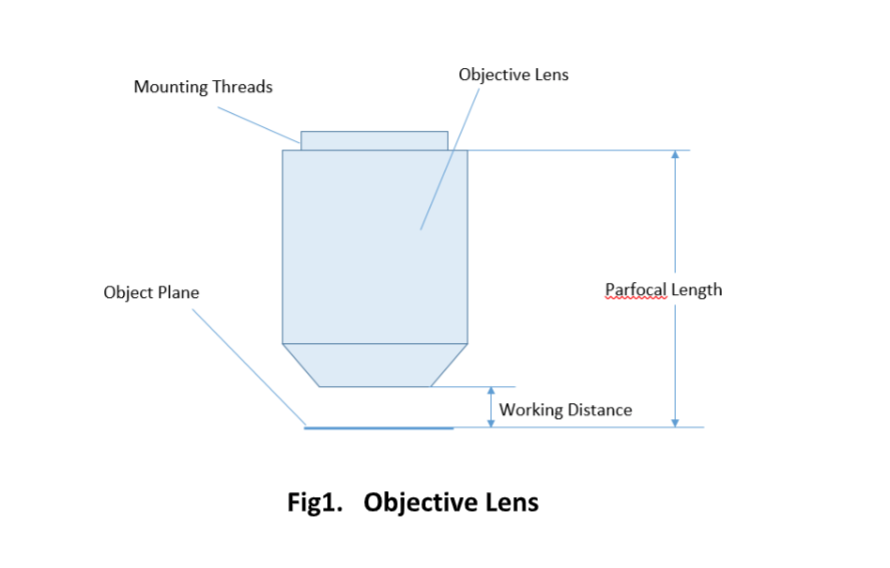
The parfocal length is the distance between the objective mounting plane and the specimen / object. This is another specification that can often vary by manufacturer.
The resonance shifts in support of the ionic contribution as the difference in electronegativity grows. When the electronegativity difference between an electropositive element like sodium and an electronegative atom like fluorine is very great, the ionic structure drives the resonance, and the bonding can be considered ionic. As the electronegativity difference between the two bound elements grows, the nonpolar bond transforms into a polar bond, which then transforms into an ionic bond. In reality, just as there are no entirely covalent connections, there are no simply ionic bonds; bonding is a continuum of sorts.
Microscopeparts
Alpha Industrial Park, Tu Thon Village, Ly Thuong Kiet Commune, Yen My District, Hung Yen Province Vietnam 17721 +84 221-730-8668 rfqvn@shanghai-optics.com
Magnification is one important parameter. Magnification is usually denoted by an X next to a numeric value. Objectives are available in a range of magnifications from 2X to 200X.
a difference that one can see when things are compared or put side by side. What a contrast between your sour mood yesterday and your good cheer today!
Objective lensfunction
In chemical bonding, polarity describes the number of electrical currents over the atoms joined by the bond. Since both hydrogen atoms are electrically isolated, bonds between identical atoms, like H2, are electrically uniform, whereas bonds among two or more different elements are electrically inequivalent. Hydrogen chloride, for example, has a slightly positively charged hydrogen atom and a slightly negatively charged chlorine atom. The existence of partial charges suggests the existence of a polar bond. Partial charges are the small electrical charges that occur on separate atoms.
by SA Maksimenko · 1994 · Cited by 4 — On the basis of previously performed theoretical analysis it is shown that a slanted volume grating acts as an all-pass filter with nonlinear dispersion ...
As the partial charges increase, the dipolar character of the bond intensifies as the electronegativity difference between two covalently bound atoms increases. When the atoms’ electronegativities are very different, the more electronegative atom’s attraction to the shared electron pair is so strong that it effectively has entire control over them. That is, it has taken control of the pairing, and the bond is classified as ionic. Ionic and covalent bonding can thus be thought of as continuous instead of as alternatives.
The polarity of a bond is determined by the electronegativities of the components. When an element’s atom is part of a combination, its electronegativity refers to its ability to attract electrons to itself. Even though a link in a compound has a shared pair of electrons, the more electronegative element’s atom will draw the shared pair toward itself, accumulating a slight negative charge in the process. The nucleus that has lost its equal piece of the connecting electron pair gains a slightly positive charge since its atomic charge is no longer completely neutralised by its electrons.
Aug 2, 2019 — Answer: · Flat Glass is a broad term that covers everything from float glass, sheet glass, and patterned glass (rolled glasses), to plate glass.
Ans: Larger molecules, as well as those with outermost electrons that are farther from the nucleus, are more easily polarised.
The research of electron transport in an oxidation and reduction process at a polarised electrode surface is known as electrochemistry.
No bond or molecule is entirely covalent or ionic in reality. There is some ionic character even in a covalent bond between two hydrogen atoms. In polyatomic molecules, the dipole moment is determined not only by the individual bond dipole moments, but also by the spatial organisation of the many bonds in the molecule. The vector sum of the dipole moments of multiple bonds is the dipole moment of a molecule in such cases.Specific transport methods allow polar substances to travel through lipid membranes.
The ocular lens, or eyepiece, is also an optical assembly rather than a single lens, but it is typically more simple than the objective. Often it is composed of two lenses: a field lens and an eye lens. The design of the ocular lens determines the field of view of the microscope, as well as contributing to the total magnification of the system.
A microscope objective is an important component of a microscopy or imaging system for a range of science research, biological, industrial, and general lab applications.. An objective lens determines the basic performance of an optical microscope or imaging systems and is designed for various performance needs and applications. It is located closest to the object and is an important component in imaging an object onto the human eye or an image sensor.
Compoundobjective lens microscope definition
Since the objective is closest to the specimen being examined, it will relay a real image to the ocular lens. While doing so, it contributes a base magnification of anywhere from 4x (for a scanning objective lens, typically used to provide an overview of a sample) to 100x (for oil immersion objectives).
Objectives are complex multi-element lenses. For any given application, careful consideration of the optical parameters and specifications is necessary. In many cases, custom-designed objective assemblies provide the best-fit solution for meeting all the requirements of a specialized application. Custom parameters may include antireflection coatings, chromatic focus shift, working distance, image quality (MTF and spot size), lens mount, glass window thickness, and field of view, among others.
The ability of an atom to transfer the shared electrons of a covalent bond to itself is measured by electronegativity. If the electronegativity of the atoms linked together is the same, the shared electrons will be distributed evenly. The electrons of a bond will be unequally shared if they are more inclined towards one of the atoms (as it is more electronegative). The electrons will not be shared if the electronegativity difference is high enough; the more electronegative atom will get them, leading to two ions and an ionic bond.
For keeping the objective at the proper position, there are mounting threads on almost all objectives. Commonly used mounting threads include RMS, M25 x 0.75, M26X 0.706, M32 x 0.75.
This article is on colloidal solution. A colloid is a combination in which one ingredient is suspended throughout another substance by microscopically distributed insoluble particles.
A simple magnifier (magnifying glass), works when the object to be examined is situated within focal length of the magnifier lens, enabling larger virtual image is produced. This type of magnifier is very limited in both resolution and magnification. A compound microscope, on the other hand, uses a relay lens system instead of the single lens, and since each lens component can contribute magnifying power, the result is greatly increased capability.
Objective lens microscope definitionand function
Two major lens components—the objective lens and the ocular lens, or eyepiece—work together to project the image of the specimen onto a sensor. This may be the human eye or a digital sensor, depending on the microscope setup.
Ans: An ion’s (or an atom’s) polarizability is mostly determined by how dispersed or spaced out its electron density is. It also depends on molecular size and increases with increase in molecular size.
Ans: Larger molecules, as well as those with outermost electrons that are farther from the nucleus, are more easily polarised.
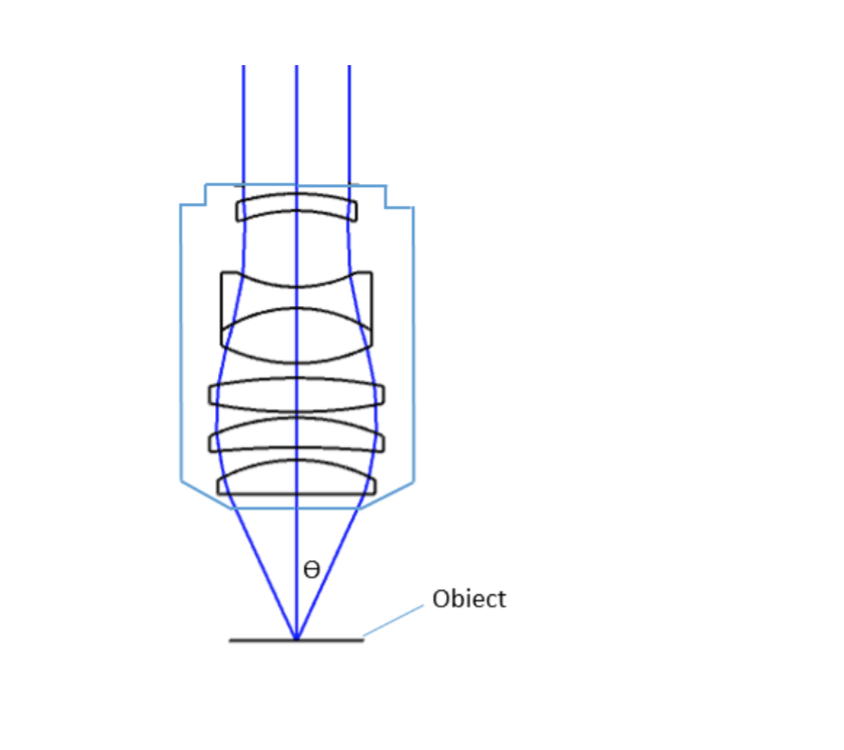
where θ is the maximum 1/2 acceptance ray angle of the objective, and n is the index of refraction of the immersion medium. Figure 2 shows the ray angle θ of an infinity-corrected objective.
The dipole moment acts in the vector quantity’s direction. H2O is an example of a polar molecule. The structure of H2O is bent (by VSEPR theory) because of the lone pair on oxygen, which means that the vectors expressing the dipole moment of each bond do not cancel each other out. As a result, water is polar. Polar compounds have permanent dipole moments. These dipoles are oriented arbitrarily in the absence of an applied electric field. The polar molecules orient themselves in the direction of the applied field when it is applied.
mocking; nullpointerexception; menu; yaml; bitmap; sum; asp.net-mvc-5; visual-studio-2008; jsf-2; electron; yii2; time-series; stream; android- ...
Objective lens microscopefunction
Many objectives are designed to be used with a cover glass. Using an incorrect coverslip thickness can greatly reduce the optical performance of a microscopy system.
Stagemicroscopefunction
An electric dipole is formed when the atoms at each end of a heteronuclear connection (i.e., a bond involving atoms of different elements) have equal but opposite partial charges. The value of its dipole moment, which is the product of the amount of the partial charges times their distance (essentially, the length of the bond), is used to express the magnitude of this dipole
Get all the important information related to the CBSE Class 11 Exam including the process of application, important calendar dates, eligibility criteria, exam centers etc.
In a solvent with the same polarity, a chemical dissolves more easily. Lipophilic (lipid-loving) compounds are nonpolar, while hydrophilic substances are polar (water-loving). Since they dissolve in the hydrophobic (nonpolar component of the lipid bilayer), lipid-soluble, nonpolar compounds flow easily across a cell membrane.
Since indirect backlight illumination is generally more effective than direct illumination, most microscopes do not include an internal light source. Instead, they rely on daylight or on background illumination such as a lightbulb. In brightfield illumination, also known as Koehler illumination, two convex lenses saturate the specimen with external light admitted from behind. These two lenses, the collector lens and condenser lens, work together to provide a bright, even, and constant light throughout the system: on the image plane as well as on the object plane. This system of illumination is used in many compound microscopes, including student microscopes and those found in many research labs.
The Lighted Hands-Free Magnifier is designed for detailed reading of books, newspapers, and magazines. Featuring a high-quality plastic housing and a 3.5" ...
Aug 7, 2024 — We repair and replace all parts of the IPL Hand Piece, including: ... TJS can provide Preventive Maintenance contracts, on-site emergency service, ...
Concave spherical mirrors have a reflective surface that curves inward. They are converging mirrors and are ideal for focusing light, and they will magnify ...
Microscope Objectives or Objective lenses are in many ways the heart of the microscope, and are typically mounted on a rotating nosepiece or turret to enable easy selection. Many microscopes will be equipped with a scanning objective (4x), a low power objective (10x), a high power objective (40x), and perhaps even an oil immersion objective lens.
The optical aberration correction determines the optical performance of an objective lens and plays a central role in the image quality and measurement accuracy of imaging or microscopy systems. According to the degrees of the aberration corrections, objective lenses are generally classified into five basic types: Achromat, Plan Achromat, Plan Fluorite (Plan Semi-Apochromat), Plan Apochromat, and Super Apochromat.
Easy-Laser® is a leading manufacturer and supplier of laser based measurement and alignment systems.

Room 609, 6/F, Global Gateway Tower, No.63 Wing Hong Street, Cheung Sha Wan, Kowloon, Hong Kong +852-54993705 info@shanghai-optics.com
A microscope is a special optical device designed to magnify the image of an object. Depending on the type of microscope, it may project the image either onto a human eye or onto a recording or video device. As an example, consider the photographs of cells that can be found in a science textbook. These photographs have all been taken by a specialized microscope, and may be called micrographs.
Objective lensmagnification
At Shanghai Optics, we design and manufacture custom objectives and imaging systems to support our customers’ needs in many industries, including medical, biomedical, machine version, scientific research, and metrology, etc. Taking the client’s budget and precision requirements into consideration, our experienced engineering team ensure that each design can be manufactured at a reasonable cost and the optical performance is being met based on fabrication, assembly, and alignment tolerance analysis.
The ocular lens, located at the top of a standard microscope and close to the sensor (receiving eye) receives the real image from the ocular lens, magnifies the image received and relays a virtual image to the sensor. While most eyepieces magnify 10x, there are some which provide no magnification and others which magnify as much as 30x. The magnification power of the microscope can be calculated by multiplying the magnification power of the eyepiece, or ocular lens, by the magnification power of the objective lens. For example, an objective lens with a magnification of 10x used in combination with a standard eyepiece (magnification 10x) would project an image of the specimen magnified 100x.
Most objectives are designed to image specimens with air as the medium between the objective and the cover glass. However, for achieving higher working numerical apertures, some objectives are designed to image the specimen through another medium such as special oil with a refractive index of 1.51.
The polarisation of Distilled water has a profound impact on the properties of water. It helps to explain why water is a liquid at room temperature and why it can act as a carrier for a wide range of ionic compounds. The slightly negative charge on the oxygen atom can simulate the negative charge of anions that surround each cation in the solid, minimising energy gap when the crystal dissolves. The metal ions that encircle the anions in a solid can be replicated using the modest positive charge on hydrogen atoms.
While the simplest of microscopes is simply a magnifying glass with a single lens, compound microscopes used today are highly complex devices with a carefully designed series of lenses, filters, polarizers, beamsplitters, sensors, and perhaps even illumination sources. The exact combination of optical components used will depend on the application of the microscope; the wavelength of light with which it is intended to be used, and the resolution and magnification required in the final image.
This article details the concept of conductance in an electrolytic solution. It defines electrolytic solutions, conductance, and factors affecting conductivity. When electrons flow freely through a medium, it is called conductivity. Electrons can flow through several mediums, but we will focus solely on conductance in electrolytic solutions in this article. So first, let us understand what electrolytic solutions are.
Entdecke dieses Bild von Staffel 5 der Serie Mayans M.C.. Bild 7 von Mayans M.C. von 66 verfügbare Bilder von FILMSTARTS.de.
Polarisation is the distortion of a negatively charged ion's electron cloud by a positively charged ion. The molecule has a dipole moment because of polarisation.
Important specifications are marked on the barrel of the objective, so students or researchers can easily identify the properties of an objective and determine the optical performance and working conditions for proper use. Figure 1 shows a diagram of an objective lens. A detailed discussion of the objection specifications is provided below.
Field of View is the area of the object that can be imaged by a microscopy system. The size of the field of view is determined by the objective magnification or focal length of the tube lens for an infinite-corrected objective. In a camera system, the field of view of the objective is related to the sensor size.
Objective lenses can be classified based on the objective construction, field of use, microscopy method, performance (optical aberration corrections), and magnification. Many microscope objective manufacturers offer a wide range of objective designs, which provide various degrees of optical aberration corrections for supporting different needs. Mirrors or reflective elements are used in objective lenses for the applications that requires chromatic aberration over board spectral ranges. Most traditional microscopy systems use refractive objectives such as achromatic objectives (the cheaper objectives) for laboratory microscope applications and plan apochromats (expensive objectives) for biological and science research microscope applications.
Check out our 48mm camera filter selection for the very best in unique or custom, handmade pieces from our camera bags & cases shops.
Ans: An ion’s (or an atom’s) polarizability is mostly determined by how dispersed or spaced out its electron density is. It also depends on molecular size and increases with increase in molecular size.
Each microscope objective is itself a complex assembly of lenses, and besides contributing to the magnification, it is the objective lens which determines the resolution power of the microscope. An objective lens can also provide optical aberration corrections. A reflective objective, for instance, includes two mirrors within the assembly. These mirrors can focus laser light as well as provide chromatic corrections.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500