Bildverarbeitungssoftware - alle Hersteller aus dem ... - bildverarbeitungssoftware
A 2-tailed t test was used to compare datafrom affected with that from unaffected contralateral eyes or normal controleyes. The Pearson correlation coefficient was calculated for the PhNR amplitudeand RNFLT.
This courseware module is offered as part of the Repository of Open and Affordable Materials at Penn State. Except where otherwise noted, content on this site is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The College of Earth and Mineral Sciences is committed to making its websites accessible to all users, and welcomes comments or suggestions on access improvements. Please send comments or suggestions on accessibility to the site editor. The site editor may also be contacted with questions or comments about this Open Educational Resource.
The scotopic threshold response (STR) is elicited by means of dim stimuli(0.6-1.0 log unit above the psychophysical threshold) and is driven by thethird-order neurons in the rod pathway.18,19 Frishmanet al20 demonstrated that the STR is eliminatedin monkeys with experimentally induced glaucoma and concluded that the STRoriginated from the ganglion cells. However, there is evidence that the STRis not eliminated in patients with traumatic optic neuropathy and in catswith optic nerve resection, which suggests that the STR mainly representsthe activity of amacrine cells.21 In clinicalpractice, it is difficult to record the STR because suitable recordings requirecomplete dark adaptation. Furthermore, patient cooperation is important duringthe recording because baseline deflection due to the blink reflex has a temporalaspect similar to that of the STR and an effect on the amplitude of the STR.22 Complex recording techniques and controversy aboutits cellular origin make the STR less valuable than the PhNR in evaluatingthe function of the ganglion cells or their axons for clinical examinations.
Atoms and molecules can absorb radiation (a photon) only if their structure has an energy difference between levels that matches the photon’s energy (hc/λ). Otherwise, the atom or molecule will not absorb the light. Once the molecule has absorbed the photon, it can either lose a photon and go back to its original lower energy level; or it can break apart if the photon energy is greater than the chemical bond holding the molecule together; or it can collide with other molecules, such as N2 or O2, and transfer energy to them while it goes back to its lower energy level. Collisions happen often, so the energy of the absorbed photon is often transferred to thermal energy.
What does it mean toabsorb the light
A red stimulus light on a blue background produced by light-emittingdiodes was used to elicit the PhNR in the previous studies, whereas a whitestimulus on a white background is recommended by the International Societyfor Clinical Electrophysiology of Vision to produce cone ERGs.6 Wehave demonstrated that a negative response, which was elicited from rats bymeans of a white stimulus on a white background, had characteristics similarto the PhNR recorded in primates and humans.7 Inaddition, the negative response was selectively reduced in patients with low-tensionglaucoma, even when the ERGs were recorded with the International Societyfor Clinical Electrophysiology of Vision protocol.8 Colottoet al9 recorded focal cone ERGs with a whitebackground in patients with POAG and showed that the negative response correlatedwith the sensitivity loss of the visual field. All of these findings indicatedthat the negative response elicited by means of a white stimulus on a whitebackground is equivalent to the PhNR elicited by means of a red stimulus ona blue background.2,5 The purposeof this study was to determine how the PhNR is affected by optic nerve atrophy.
What substance absorbslightenergy during photosynthesis
Gotoh Y, Machida S, Tazawa Y. Selective Loss of the Photopic Negative Response in Patients With OpticNerve Atrophy. Arch Ophthalmol. 2004;122(3):341–346. doi:10.1001/archopht.122.3.341
We found a good correlation between the reduction of the PhNR amplitudeand RNFLT at the advanced stages of the disease. However, as shown in Figure 3 and Figure 4, PhNR amplitude loss preceded RNFLT thinning at 1 and 3months after disease onset. This discrepancy could be due to several factors.First, excessive glutamate released by optic nerve damage can lead to PhNRloss because of suppression of the synaptic transmission between the bipolarand ganglion cells. The N-methyl-D-aspartateand α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptorswere involved in ganglion cell death after the optic nerve was crushed inrats.26 In addition, we previously reportedthat intravitreal injection of N-methyl-D-aspartate agonist eliminates the PhNR in rats.7
Results The amplitudes of the rod, maximum, cone, and 30-Hz flicker electroretinogramsof the affected eyes were not different from those of the corresponding wavesof the contralateral unaffected eyes or control eyes. In contrast, the amplitudeof the PhNR was significantly smaller in the affected eyes than in the contralateral(P = .005) or control (P<.001)eyes. The decrease in amplitude of the PhNR preceded thinning of the retinalnerve fiber layer. There was a significant correlation between the PhNR amplitudeand retinal nerve fiber layer thickness in eyes with optic nerve atrophy (r = 0.879; P<.001).
Methods Ten patients with optic nerve atrophy induced by compression, trauma,or inflammation were examined. There were 6 men and 4 women with a mean ageof 52.4 years. Ten age-matched control subjects were examined with the sameprotocol. Full-field electroretinograms were recorded, and the retinal nervefiber layer thickness surrounding the optic nerve head was measured by meansof optical coherence tomography.
Absorb the lightanswer
All of the data of the photopicnegative response (PhNR) amplitude and retinal nerve fiber layer thickness(RNFLT) from 12 affected and 8 unaffected eyes in the 10 patients were plotted.The PhNR amplitude and RNFLT were measured more than 6 months after diseaseonset (r = 0.879; P<.001).
The John A. Dutton Institute for Teaching and Learning Excellence is the learning design unit of the College of Earth and Mineral Sciences at The Pennsylvania State University.
Representative electroretinogramsrecorded from patient 2 at 6 months after symptom onset of compression opticneuropathy. The photopic negative response (PhNR) amplitude (arrows) was measuredfrom the baseline to the trough between the cone b-wave and the i-wave.
Transmission oflight
Representative ERGs recorded from the affected and unaffected contralateraleyes in patient 2 at 6 months after symptom onset of compression optic neuropathyare shown in Figure 1. There wasno difference in the waveforms of the scotopic rod, maximum, and 30-Hz flickerERGs between the affected and unaffected eyes. However, PhNR was not presentin the cone ERG of the affected eye, but a distinct negative deflection ofapproximately 40 µV following the b-wave was present in the unaffectedeye.
Reduction of the PhNR is correlated with the loss of sensitivity ofthe visual field in patients with POAG.5 Ourresults provide additional evidence that the PhNR is strongly attenuated bydiseases of the optic nerve other than POAG.
The patients underwent comprehensive ophthalmological examination includingSnellen visual acuity, slitlamp biomicroscopy, indirect ophthalmoscopy, andkinetic or static visual field perimetry. The left eyes from 10 age-matchedcontrol subjects were examined with the same protocol for the ERG recordings.
Absorption of energy is called
Conclusions Selective reduction and loss of the PhNR amplitude was found in eyeswith optic nerve atrophy, which suggests that the PhNR can be used to evaluatethe function of ganglion cells or their axons.
The absorption cross section, σ, varies significantly over the width of the absorption line. So it is possible for all the radiation to be absorbed in the middle of the line but very little absorbed in the “wings.”
The PhNR is strongly attenuated in primate eyes with experimentallyinduced glaucoma and also in eyes injected with tetrodotoxin,2 ablocker of the neural activity of retinal ganglion cells, their axons, andamacrine cells.3,4 The amplitudeof the PhNR was reduced in patients with primary open-angle glaucoma (POAG),and the reduction was correlated with the optic nerve damage represented byoptic disc cupping and visual field loss.5
We recently demonstrated that the first-order kernel of the multifocalERG contains a component probably originating from ganglion cells.17 During specific conditions of stimulation, such asa low temporal frequency and 50% contrast, a small positive wavelet appearson the descending part of the first positive wave (P1) and is designated thes-wave. This response is strongly attenuated in patients with optic neuritisand is correlated with psychophysiological functional measures, such as criticalflicker frequency and visual acuity. Measuring s-wave amplitude has a greatadvantage over measuring PhNR amplitude in evaluating focal functional lossof ganglion cells. However, multifocal ERG is not practical in patients withpoor visual acuity and opacities of the ocular media because unstable fixationand distortion of the retinal image affect multifocal ERG. Because full-fieldERGs are not generally affected by these factors, PhNR is better than multifocalERG for evaluation of ganglion cell function in selected patients with severeloss of visual acuity and with opacities in the ocular media.
Opacities of the ocular media have a great effect on results measuredby means of visual acuity and visual field. Total hyphema could be one ofthe causes of a relative afferent pupillary defect,13 whichis widely used as a sign of unilateral optic nerve dysfunction. Furthermore,corneal opacities prevent a precise assessment of the pupillary reflex. Incontrast, the waveform of the cone ERGs produced by means of a strong flashare not affected by opacities of the ocular media, which suggests that PhNRamplitude could be used to evaluate residual optic nerve function and determinesurgical indications in cases of severe opacity of the ocular media.
The PhNR amplitude was normal at 1 week after symptom onset, even thoughvisual acuity had decreased to light perception. At 1 month after onset, thePhNR amplitude was considerably reduced, but the RNFLT was only slightly thinner.This finding suggests that functional loss preceded RNFLT thinning (Figure 3).
It is generally believed that the activity of the third-order retinalneurons contributes little to the shaping of the corneal electroretinogram(ERG). However, a response has been newly identified that originates fromthe third-order neurons that receive signals from cones.1 Thisresponse was named the photopic negative response (PhNR),2 andit consists of a negative-going wave that appears immediately after the coneb-wave (Figure 1).
All of the data of the PhNR amplitude and RNFLT from the 12 affectedand 8 unaffected contralateral eyes in the 10 patients were plotted to determineif a correlation existed between PhNR amplitude and RNFLT (Figure 5). The PhNR amplitude and RNFLT were measured more than6 months after symptom onset when optic nerve atrophy is probably complete.The reduction of PhNR amplitude significantly correlated with RNFLT thinningat this time (r = 0.879; P<.001).The PhNR amplitudes of the affected eyes did not overlap with those of theunaffected contralateral eyes (Figure 5).We also found a significant correlation between the PhNR amplitude measuredafter the i-wave and the RNFLT (r = 0.902; P<.001; data not shown).
The College of Earth and Mineral Sciences is committed to making its websites accessible to all users, and welcomes comments or suggestions on access improvements. Please send comments or suggestions on accessibility to the site editor. The site editor may also be contacted with questions or comments about this Open Educational Resource.
Except where otherwise noted, content on this site is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
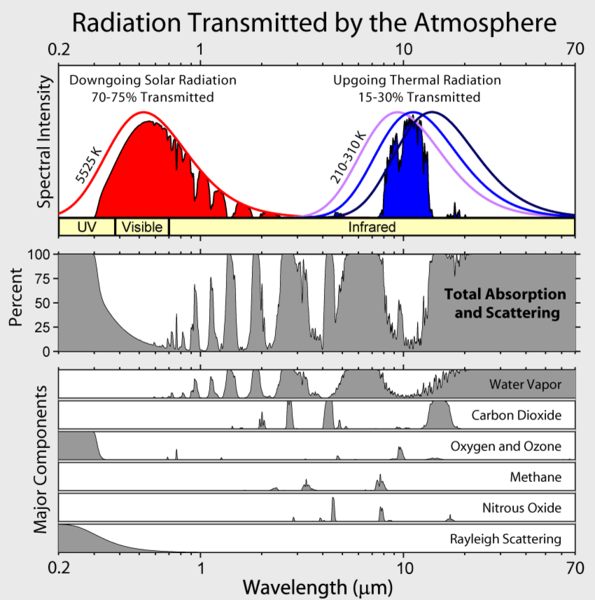
Note that Earth's outgoing infrared irradiance is limited to a few atmospheric "windows" and the irradiance at all other wavelengths is strongly absorbed, mostly by water vapor, but also by carbon dioxide, ozone, nitrous oxide, methane, and other more trace gases that aren't shown in the figure above.
Fundus photographs (A), opticalcoherence tomographic images (B), and cone electroretinograms (C) at 1 weekand 1, 3, and 6 months after symptom onset in patient 4 with compression opticneuropathy. A, Color photographs of the optic nerve head are shown. B, Theretinal nerve fiber layer thickness (RNFLT) around the optic nerve head wasmeasured. The representative values are the mean RNFLT of the 100 points alonga 360° path around the optic disc. The arrowheads indicate the retinalnerve fiber layer. NAS, indicates nasal; SUP, superior; TEMP, temporal; andINF, inferior. C, Photopic negative response (PhNR; arrows) is shown.

The amplitudes of the rod, maximum, cone, and 30-Hz flicker ERGs foreach patient are shown in Table 1.All of the ERG recordings were obtained more than 6 months after symptom onsetand at a time when the optic nerve head appeared atrophic. The amplitudesof the scotopic rod b-wave, maximum scotopic a-wave and b-wave, cone a-waveand b-wave, and 30-Hz flicker ERGs of the affected eyes did not differ fromthe corresponding components in the unaffected contralateral eyes. On theother hand, the amplitudes of the PhNR were significantly reduced in the affectedeyes (patients 1-10), as compared with those in the unaffected contralateraleyes (patients 1-4, 6-8, and 10; P = .005) and normalcontrol eyes (P<.001) (Figure 2).
Changes in visual acuity cannot be used to quantify optic nerve function.Visual acuity can be well preserved if the papillomacular bundle of the nervefiber is not impaired, even if widespread damage to the optic nerve is present.Although Thompson et al14 described a methodto quantify relative afferent pupillary defect by using neutral density filters,26% to 50% loss of ganglion cells is necessary to cause relative afferentpupillary defect.15,16 This findingindicates that pupillary reflexes are not a sensitive estimate for detectingoptic nerve dysfunction.
To answer this question, we need to look at the configurations of the electrons that are zooming around atoms and molecules. More than 100 years ago, scientists began using prisms to disperse the light from the sun and from flames containing different elements. While the sun gave the colors of the rainbow, the flames had light in very distinct lines or bands. This puzzle was finally resolved a little more than 100 years ago with the invention of quantum mechanics, which basically says that the electrons zooming around atoms and molecules and the vibrations and rotations of molecules can have only discrete energies that are governed by rules of conservation of angular momentum.
In the present study, we measured PhNR amplitudes before and after thei-wave. Both measurements produced similar results, which indicates that eitherscoring method is useful to evaluate the remaining function of ganglion cellsand their axons in optic nerve atrophy.
Second, glial alterations induced by optic nerve damage may reduce thePhNR amplitude. Experimental evidence suggests that glial mediation generatesPhNR: intravitreal injection of barium blocks the potassium current in glialcells, with the subsequent elimination of PhNR in cats.27 Glialcell changes also occur after optic nerve resection.28 Allof this evidence suggests that glial involvement could contribute to reductionof PhNR at the early stage of disease.
There was a significant correlation between RNFLT thinning and PhNRamplitude loss in patients with optic nerve atrophy (Figure 4). This finding indicates that PhNR is a good measure ofthe surviving ganglion cells and their axons at the advanced stage of opticnerve disease. In addition, PhNR amplitudes in diseased eyes were not withinthe normal range of unaffected contralateral or normal control eyes, whichsuggests that PhNR amplitude measurement can be used to differentiate diseasedeyes with optic nerve atrophy from normal ones.
After the pupils were maximally dilated with topical 0.5% tropicamideand 0.5% phenylephrine hydrochloride, all subjects underwent dark adaptationfor at least 30 minutes. After topical anesthesia was induced with oxybuprocainehydrochloride, the contact lens electrode, containing light-emitting diodes(EW-102; Mayo, Nagoya, Japan), was inserted in dim red illumination. The light-emittingdiodes provided homogeneous white (color temperature, 4000-9000 K) stimulusand background light. Luminance produced by the contact lens electrode atthe corneal side was measured with a photometer (LS-100; Minolta, Tokyo, Japan).Details of the contact lens electrode have been previously described.10 The reference and ground electrodes were placed onthe forehead and right earlobe, respectively.
What happens whenlightis absorbed
After a full explanation of the nature of the study, informed consentwas obtained from all patients. The procedures used in this study conformedto the tenets of the Declaration of Helsinki.
5 things thatabsorb light
Selective reduction and loss of the PhNR amplitude was found in patientswith optic nerve atrophy. The presence and size of PhNR was used to discriminatebetween patients with optic nerve atrophy and healthy control subjects, whichsuggests that PhNR can be used to evaluate the function of ganglion cellsor their axons. The PhNR seems to be a good measure for quantifying RNFLTloss in eyes with optic nerve atrophy.
In our previous article,8 conventionalcone ERGs were recorded in patients with low-tension glaucoma, and no correlationwas found between the loss of sensitivity of the visual field and PhNR amplitudereduction. In contrast, Viswanathan et al5 demonstratedthat PhNR amplitude was significantly correlated with visual field sensitivity,with a moderately high coefficient of correlation in patients with POAG. Thesefindings imply that the i-wave intrusion in our study may prevent an accuratemeasurement of PhNR amplitude, although we cannot eliminate the possibilitythat the alterations in eyes with low-tension glaucoma may have contributedto the difference.
Our results showed that PhNR amplitude remained unchanged in the earlystages of optic neuropathy, even though vision had already been severely impaired.However, PhNR amplitude was reduced or completely lost as RNFLT thinning progressed.This finding indicates that PhNR does not reflect an early functional lossof the optic nerve without retinal structural change. Therefore, PhNR is nota suitable component to evaluate optic nerve function during the early stageswhen the normal structure of the ganglion cells and RNFLT are still preserved.Measuring visual acuity and visual fields and assessing pupillary light reflexesare quicker and easier than recording ERGs during the early stages.
From the Department of Ophthalmology, Iwate Medical University Schoolof Medicine, Morioka, Iwate, Japan. The authors have no relevant financialinterest in this article.
Changes in the mean of the retinalnerve fiber layer thickness (RNFLT) and photopic negative response (PhNR)amplitude in patients 2, 4, 7, and 8 after disease onset. Each value at 1,3, and 6 months is expressed as the percentage of RNFLT or PhNR amplitudeseen at 1 week. Error bars indicate standard error of the mean.
Averaged values of the relative RNFLT and PhNR amplitudes in patients2, 4, 7, and 8 are plotted against the time after symptom onset in Figure 4. The values of the RNFLT and thePhNR amplitude at 1 week after the onset were set at 100%. There was a largediscrepancy between the loss of RNFLT and PhNR amplitude at 1 month. Althoughthe RNFLT was preserved for the first month after the onset, the PhNR amplitudewas significantly reduced (P<.01). Reduction ofthe RNFLT progressed until 3 months, after which there were no further changes.
© 2024 American Medical Association. All rights reserved, including those for text and data mining, AI training, and similar technologies. Terms of Use| Privacy Policy| Accessibility Statement| Cookie Settings
Corresponding author and reprints: Shigeki Machida, MD, Departmentof Ophthalmology, Iwate Medical University School of Medicine, 19-1 Uchimaru,Morioka, Iwate 020-8505, Japan (e-mail: smachid@clubAA.com).
In previous studies, red stimuli on a blue background were used to recordthe PhNR.2,5 This stimulus configurationwas advocated because it enhances the PhNR amplitude. However, with whitestimuli on a white background in the present study, the PhNR amplitude rangedfrom 28 to 69 µV with a mean of 43 µV in normal control eyes.This finding indicates that even a conventional white stimulus on a whitebackground will elicit substantial PhNR amplitude as long as the recordingsarise from the photopic system.
The pattern ERG is another response that reflects the activity of ganglioncells and their axons.23,24 Thecellular origin of negative potentials (N95) of the pattern ERGis similar to that of the PhNR.25 However,from a practical standpoint, full-field ERGs are easier to record than arepattern ERGs because they do not require refractive correction, clear ocularmedia, and exact foveal placement.
Absorb the lightmeaning
However, we noted a difference in the cone ERG waveform between previousrecording conditions and ours. In our recordings, the PhNR was followed bythe i-wave that obscured the trough of the PhNR. The i-wave contributed littleto the cone ERG in previous recording conditions, which then allowed a clearobservation of the trough of the PhNR. The i-wave represents mainly the offresponse that grows in amplitude with light adaptation. A narrow-wavelengthband of blue background light adapts the rods but not the cones so that thei-wave is not enhanced by blue light adaptation.
Averaged amplitudes of the photopicnegative response (PhNR) from normal control, affected, and unaffected contralateraleyes 6 months after disease onset. Error bars indicate SEM.
The following bulleted list is a crash course in absorption by the electrons in atoms and molecules. Refer to the figure below the box.
The amplitudes of the PhNR varied considerably in the normal controleyes.5 In our control subjects, the PhNR amplitudesranged from 28.1 to 68.7 µV, but none of the values from the affectedeyes was within this range (Table 1).The amplitude of the PhNR measured after the i-wave was also significantlyattenuated in the affected eyes, as compared with that in the unaffected contralateraleyes (P = .001) and normal control eyes (P<.001; data not shown).
The atmosphere absorbs a significant amount of radiation in the infrared but rather little in the visible. Also, we see that gases absorb strongly at some wavelengths and not at others. Why is this?
Focal defects in the retinal nerve fiber layer can be detected withoptical coherence tomography in early to moderate stages of glaucoma.11,12 We were able to measure the RNFLTand the PhNR amplitude in patients 2, 4, 7, and 8 from the early stages tothe advanced stages in which optic disc atrophy was complete. The fundus photographsand optical coherence tomographic images obtained at 1 week and 1, 3, and6 months after symptom onset are shown for patient 4 in Figure 3. This patient had compression optic neuropathy.
After 30 minutes of dark adaptation, rod and maximum ERGs were producedwith white stimuli of 0.5 and 3.3 log cd/m,2 respectively,with 3-millisecond duration. Then the cone ERGs and 30-Hz flicker ERGs wereproduced with 3.0 log cd/m2 with 3-millisecondduration and 2.0 log cd/m2 with 33.3-millisecondduration, respectively, with a diffuse white background of 40.0 cd/m2. Subjects underwent light adaptation by means of the white backgroundlight of 40.0 cd/m2 for at least 10 minutesbefore the photopic recordings. The stimulus intensity and duration of thelight-emitting diodes were controlled by a stimulator (WLS-20, Mayo). Responseswere amplified at 5000 gain and a band-pass filtered from 0.5 to 1000 Hz (NeuropackMED 5210; Nihon Kohden, Tokyo, Japan). Three to 16 responses were computeraveraged with flash intervals of 2 seconds and 2 minutes for the rod and maximumERGs, respectively. Thirty-two responses were averaged for the photopic recording.
Ten patients with optic nerve atrophy induced by compression (n = 5),trauma (n = 3), or inflammation (n = 2) were examined (Table 1). The patients included 6 men and 4 women whose ages rangedfrom 39 to 67 years with a mean of 52.4 years. Compression optic neuropathywas attributed to surgically resected pituitary adenoma or sphenoidal cystocele.The patients with traumatic optic neuropathy had been treated with systemiccorticosteroids without decompression surgery. Optic neuritis was associatedwith multiple sclerosis in patient 9, but the cause could not be determinedin patient 10. Both patients were treated with systemic corticosteroids withimprovement of visual acuity and visual field but not of optic nerve atrophy.
After the pupils were fully dilated, fundus photographs were obtainedin all patients with a fundus camera (IA-50; Topcon Corp, Tokyo, Japan). Wesubjectively compared the disc color of the affected eyes with that of theunaffected contralateral eyes to determine whether optic nerve atrophy waspresent.
The a-wave and b-wave amplitudes were measured from the baseline tothe trough of the first negative response and from the first trough to thepeak of the following positive wave, respectively. In previous articles,2,5 the PhNR was defined as a negative-goingwave immediately after the cone b-wave. The i-wave became apparent and obscuredthe negative trough of the PhNR in light-adapted conditions. The negativetrough of the PhNR appears before or after the i-wave (Figure 1). We measured the PhNR amplitude from the baseline to thenegative trough between the cone b-wave and the i-wave, as well as just afterthe i-wave. However, the amplitude of the PhNR measured between the cone b-waveand the i-wave is shown in the figures and table. Although the PhNR is a negative-goingwave, its amplitude was expressed as a positive value in this study.
An optical coherence tomography scanner (OCT 2000; Carl Zeiss Meditech,Dublin, Calif) was used to obtain cross-sectional tomograms of the eyes. Thedelay of the light backscattered from the different layers in the retina wasdetermined by using low-coherence interferometry. To measure the retinal nervefiber layer thickness (RNFLT) around the optic nerve head, we used circularscans 2.0 mm in radius (OCT Application Version A6.1, Carl Zeiss Meditech).Each image consisted of RNFLT measurements at 100 points along a 360°path around the optic disc. A mean RNFLT of the 100 points was used for theanalysis.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500