RGB vs. RGBW vs. RGBIC vs. RGBWW vs. RGBCCT LED ... - led lights rgb
By submitting the information, you give your consent to the potential publication of your inputs on our website according to our rules. (If you later retract your consent, we will delete those inputs.) As your inputs are first reviewed by the author, they may be published with some delay.
Light is also a type of electromagnetic wave. When using light in machine vision, wavelength and the corresponding photon energy are important factors to consider. Different light phenomena occur depending on the wavelength, which makes it possible to highlight and thus capture the target information. You should avoid looking directly into the light source as much as possible when using the light, especially when using ultraviolet (UV) and short-wavelength visible light (violet, blue, etc.).
In some special cases, nearly all of the absorbed light causes fluorescence rather than heat, and there can be even a net cooling effect (→ laser cooling). It may even happen that at some (typically longer) wavelengths one obtains laser amplification for strong enough excitation of the medium, usually involving a population inversion. The medium may then generate laser radiation which may remove a substantial fraction of the deposited energy.
Notes for reflection oflight
For example, an incandescent bulb works based on the principle that light is emitted when the energy level of electrons in numerous atoms changes from a high level EH to a lower level EL after being excited to the high level by the injection of electrical energy.
A general distinction is between intrinsic and extrinsic absorption. Extrinsic absorption (also sometimes called parasitic absorption) results from things which could in principle be avoided – for example, from impurities and structural defects which could be absent in pure high quality material. Intrinsic absorption results from basic properties of the pure material.
One photonâs energy is extremely small for humans to perceive, but innumerable photons gathered together (in the visible wavelength range) allow humans to sense "brightness."
Various types of processes, which would in principle be avoidable, lead to extrinsic absorption for example in optical glasses, in nonlinear crystal materials and in laser crystals:
Water absorption coefficient
Absorption in a semi-transparent medium is usually quantified with an absorption coefficient, telling which fraction of the optical power is lost per unit length. The inverse of an absorption coefficient is called an absorption length. The absorption of a given length of material (e.g. of a plate with a certain thickness) can be quantified with an absorbance.
Non-transparent objects can be attributed an absorptance, which is the fraction of incident light which is absorbed rather than transmitted, reflected or scattered.
Here you can submit questions and comments. As far as they get accepted by the author, they will appear above this paragraph together with the author’s answer. The author will decide on acceptance based on certain criteria. Essentially, the issue must be of sufficiently broad interest.
The sun emits various types of electromagnetic waves. Harmful ones that have wavelengths shorter than 280 nm (γ (gamma) rays, X-rays, UV-C, etc.) do not reach the Earthâs surface because they are mostly absorbed by the atmospheric layer above the earth. One of the reasons astronauts wear spacesuits outside the spacecraft is to protect their bodies from the harmful electromagnetic waves like gamma rays, X-rays, and UV rays above the atmosphere.
If absorption of light causes heating of the absorbing medium, that will subsequently lead to thermal expansion. The heating is often strongly inhomogeneous; for example, it may occur within a focused laser beam. The local thermal expansion then leads to mechanical stress in the medium, which can even result in fracture when the deposited thermal power or energy is sufficiently high. Further, the temperature causes a slight local modification of the refractive index, which (together with stress-related effects) can cause thermal lensing effects.
The above focuses on one electron. When such a phenomenon occurs with a large number of atoms, numerous quanta (photons) of electromagnetic energy will be released one after another by the transition of each electron, and vibrate with its wavelength corresponding to each energy as it moves through space.
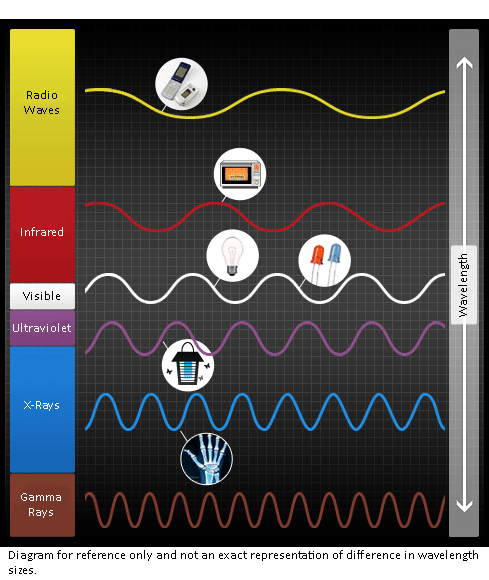
ã»Radio waves: short-, medium-, and long-wave types are used for radio and TV broadcastingã»Microwaves: used in microwave ovensã»Light: ultraviolet, visible light, and infraredã»X-rays: used for medical imaging
Conversely, when light with a certain wavelength (frequency) interacts with atoms, the light gives its energy to electrons if certain conditions are met.
When the magnetic field changes, it exerts force on electric charges to move them (current flow), and when current flows, it changes the magnetic field (electromagnetic induction). Synchronized oscillations of electric and magnetic fields are created by the interaction of the two, and electromagnetic energy propagates as a transverse wave in space.
Electromagnetic waves have both wave-like properties and particle-like properties, illustrated in the examples below. This property is one of the central principles of modern quantum mechanics.
Absorption of light can also have electrical effects. For example, there are photoresistors, where the electrical resistance is reduced by absorbed light. In photodiodes and phototransistors, one exploits the internal photoelectric effect, related to the excitation of electric carriers by light absorption.
Each photon carries energy and collides with various objects, including the human eye and skin. High-energy photons can change or destroy the molecular structure of an object. This is why shorter wavelengths like γ (gamma) rays and X-rays are hazardous and cause severe damage to the living tissues of humans and animals. In the case of non-living objects such as paper or cloth, for example, it may cause deterioration or discoloration.
There are also many cases where a material contains some absorbing dopant while the host material itself exhibits only negligible absorption. This is the case for solid-state (doped-insulator) gain media.
As light carries energy, the absorption of light is associated with the deposition of energy in the absorbing medium. In most cases, that energy is mostly converted into heat, although sometimes a substantial amount of the received energy is radiated away as fluorescence.
The energy of photons is inversely proportional to the wavelength. Photons have higher energy as the wavelength becomes shorter. âª3â«
Electromagnetic waves with long wavelengths (infrared or radio waves) have less photon energy, which makes them less harmful to the body, but that does not mean there is no danger at all. For example, glassworkers can develop cataracts over time due to infrared radiation from extreme heating of the glass. All of the above examples of negative effects on the living body and objects are related to the wavelength dependence and intensity of electromagnetic energy.
Electromagnetic waves with wavelengths in the UV-C range are extremely damaging to living organisms. Furthermore, UV-B and UV-A that partially reach Earth are not completely safe.
When the energy state of the electron changes from high energy (EH) to low energy (EL), the energy difference (ÎE = EH-EL) will be released from the atom as wave energy. This is light itself (electromagnetic waves). The photon is the smallest unit (quantum) of the wave energy that forms electromagnetic waves.
Saturable absorption can also be considered as a kind of nonlinear absorption. Here, however, the absorption coefficient is reduced under the influence of intense light, e.g. because the starting electronic level for the light absorption is depleted.
Visiblelightwavelength
Electromagnetic waves are a physical phenomenon in which energy vibrates between an electric field and a magnetic field. It includes wavelengths that are much shorter than light such as γ (gamma) rays and X-rays, as well as much longer wavelengths including microwaves and radio waves.
Even simple linear absorption processes introduce some amount of quantum noise. This can be intuitively understood by considering that some of the incident photon are randomly removed, while other photons remain in the light beam. An initially perfectly regular stream of photons (→ amplitude-squeezed light) would thus be converted into a random stream of photons, exhibiting some intensity noise.
All electromagnetic waves are composed of photons. A photon is the smallest discrete amount or âquantumâ of electromagnetic radiation. It is the basic unit of all light.
The physical existence of this website has been verified by using a sever certificate issued by Cybertrust. Additionally, encryption is used to protect the privacy of communications made via SSL webpages.
UV-B is the main cause of damage that results from extended exposure to strong direct sunlight, including sunburns, skin cancer, and even damage to DNA. It also causes a type of eye inflammation called snow blindness, where UV rays reflected off snow give eyes sunburn. Although UV-A is less dangerous than UV-B, it can reach deep inside the skin, denaturing collagen that makes up the skinâs tissues and promoting skin aging like spots and freckles.
Even wavelengths longer than UV can cause damage. An intense blue light or overexposure to it carries the risk of damaging your retina when directly looking at it.
Mass absorption Coefficients
Electromagnetic waves are a physical phenomenon in which energy vibrates between an electric field and a magnetic field.
A pebble thrown into a pond creates ripples. An object floating on the water vibrates up and down with the ripples but stays in place (unless moved by an outside force like wind). The wave energy propagates to the surroundings along with the ripples.
The term absorption is not only used for absorption processes, but also often for related quantities, e.g. instead of absorption coefficient.
Sunlight can turn white paper yellow over time because the photon energy of shorter wavelengths damages the paperâs molecular structure. The damage makes it harder for the paper to reflect shorter wavelengths in the visible light range, thus changing its color.
Lightabsorption
X-ray imaging usually does not cause any problem for humans since the X-ray irradiates only for a short time, but there is a high risk for X-ray technicians who are repeatedly exposed, which is why they take the image from a separate room.
Further, the modified population in electronic states can substantially modify the absorption at the wavelength of the absorbed light and also at other wavelengths. It has already been mentioned above that absorption may be saturated. In other cases, light absorption is strongly increased by the light-induced changes in the state of matter. That is often exploited in laser material processing, where the initial absorption e.g. by a metal is weak, but strongly increases once the material is strongly excited (anomalous absorption). In various materials, one may obtain excited-state absorption at wavelengths where the material would normally not be absorbing. In semiconductors, at high intensities one obtains free carrier absorption.
Last time, we explained that light is one form of electromagnetic waves, and the concept of light, which is based on human sense, was historically expanded to the broader concept of ultraviolet, visible, and infrared as physical energy of the electromagnetic waves.
Please do not enter personal data here. (See also our privacy declaration.) If you wish to receive personal feedback or consultancy from the author, please contact him, e.g. via e-mail.
An electric field is a property of space that surrounds an electric charge and exerts force on other charges in the field. There are two types of electric charge: positive (+) and negative (-). Like charges repel each other and opposite charges attract each other.
On the other hand, most visible and infrared light reaches the Earth after being partially absorbed by atmospheric moisture. Life on Earth can exist thanks to solar energy from the visible and infrared range while the atmosphere blocks shorter wavelengths that harm the body.
The oscillations of the electric field H and the magnetic field E are perpendicular to each other and perpendicular to the direction of energy and wave propagation Z.
In nonlinear absorption, does the laser pulse duration also affect the absorption coefficient alongside with the intensity?
Everything in nature is made up of atoms, and atoms are made up of nuclei and electrons as shown on the right. The electron orbits the nucleus in multiple energy states. This is only an approximate model as the electron orbits are probabilistic in nature.
Another phenomenon called wave diffraction occurs when waves encounter an obstacle and bend around it. The longer the wavelength, the greater the bend around the obstacle.
Are you still registered with CCS members? If you register as a CCS member, you will be able to log in and register with the CCS members, download various materials (drawings, instruction manuals etc), select "lighting selection", "apply for lending machine", " It becomes possible to browse and download all contents of our site including request of "quotation" and "catalog", and it will be possible to use many convenient functions. Come and register.
The energy imparted by the light causes electrons to move to a higher level. This is called excitation of the energy state of electrons due to the light. The phenomenon where light energy is transferred to electrons is called absorption of light. For example, when putting a white paper and a black paper under direct sunlight, the black one gets higher in temperature because it absorbs more light energy.

As absorption coefficients are wavelength-dependent, one often produces absorption spectra, showing an absorption coefficient as a function of wavelength or optical frequency.
Light absorption processes e.g. in solid materials generally arise from the interaction of the electromagnetic wave with electrons, exciting those to excited energy levels. Thereafter, it takes some time (the electron–lattice thermalization time) for that energy to be transferred to the atomic nuclei, i.e., to vibration energy. That typically happens within a couple of picoseconds, and thereafter it takes far longer times to distribute that heat over some volume of the medium. That means that the thermalization, let alone the heat conduction, can take far more time than the pulse duration of a femtosecond laser. That has important implications for laser material processing with ultrafast lasers, where the involved processes cannot be understood as simply heating up the material. Instead, one is dealing with highly non-equilibrium states of matter, which can lead to rapid application of material while very nearby other material, not directly hit by the laser radiation, is not even significantly heated.
The ripples on the water are reflected in the opposite direction after hitting the shore. The incoming waves and the returning waves overlap, which can strengthen or weaken each other. This is called the wave interference phenomenon. The object floating on the water irregularly vibrates up and down based on the overlapping of the waves.
Furthermore, if the wavelength of light is λ [m] (read as âlambdaâ), and the frequency is ν [1/s], the traveling speed c [m/s] is
If the incident light is in a coherent state, exhibiting the standard shot noise level, the extra noise added through linear absorption is just enough to keep the residual light at the shot noise level (which is relatively stronger for weaker light).
For example, electromagnetic waves exhibit both the wave diffraction and interference phenomenon, which becomes more prominent as the wavelength gets longer. The radio and television waves sent from broadcasting stations can be received even in the shadows of buildings or indoors because their long wavelength causes greater diffraction.
If absorption is caused by some absorbing dopant, the contribution to the absorption per dopant atom or ion is often quantified with an absorption cross-section.
Linear absorption means that the absorption coefficient is independent of the optical intensity. There are also nonlinear absorption processes, where the absorption coefficient is a linear or higher-order function of the intensity. For example, two-photon absorption is a process where two photons are absorbed simultaneously, and the absorption coefficient rises linearly with the intensity. Multiphoton absorption processes of higher order are often involved in laser-induced damage caused by intense laser pulses.
If light is absorbed by atoms or molecules of a gas, light forces associated with the absorption may become relevant. They can be used for Doppler cooling, for example.
Through different kinds of processes, which are explained in the following, light can be absorbed in various media. This implies that the optical energy is converted into some other form of energy (but sometimes back again to optical energy). In most cases, the energy is eventually transformed into heat (thermal energy).
About 0.5% of UV-B (280-315 nm) and 5% of UV-A (315-380 nm) are said to reach the surface depending on the season and weather. One exception is the ozone hole above the Antarctic region. It is regarded as a serious environmental concern because ultraviolet (UV), which should be absorbed in the ozone layer, passes through the ozone hole and reaches the Earth.
More specific terms: infrared absorption, excited-state absorption, pump absorption, light-induced absorption, multiphonon absorption, multiphoton absorption, two-photon absorption, pump absorption
Impurities can also modify intrinsic absorption features – for example, shift the band gap energy and the corresponding absorption edge when a semiconductor compound is formed.
As wavelength gets shorter, the wave nature becomes less prominent, and the electromagnetic waves will travel straight ahead with little diffraction, behaving more like a particle. âª2â« This can be seen with visible light. Although it has both wave and particle properties, its shorter wavelength appears to move linearly and thus causes the appearance of shadows. However, its diffraction effect is visible when using certain experimental devices.
This time, we would like to talk about the basic properties of electromagnetic waves and the common properties of light.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500