LED Dome Light Set - custom dome lights
Does this quantum energy model agree with classical mechanics? Yes. If you looked at a tennis ball bouncing back and forth in a typical classroom, you could calculate the quantized energy levels. However, these energy levels are so close to each other that you essentially would never be able to experimentally verify that the ball can only have certain energy levels.
What about the photoelectric effect? Well, all the results you see experimentally can be explained if the electrons in the metal can only exist at certain energy levels (quantum model of matter) and the light is a wave. Actually, some of the older quantum mechanics textbooks show this as an example problem.
Optical microscopemagnification
These are one form of Maxwell’s equations. They describe the relationship between the electric and magnetic field (well mostly the last two). If you like, you can use vector calculus on the above equations and then eliminate B to get:
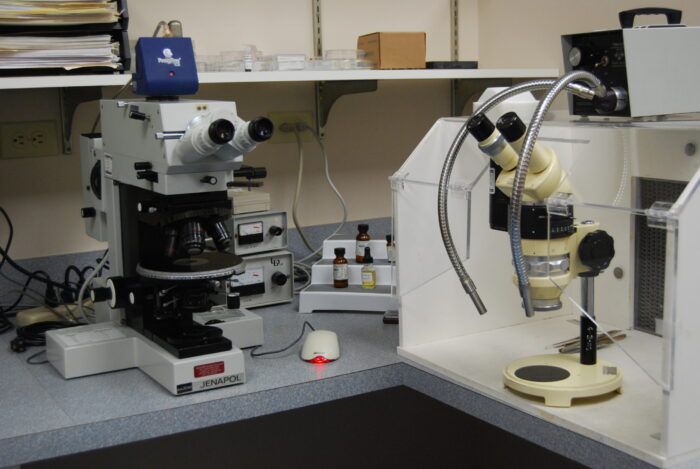
Light microscopy analysis is an important tool in particle and material characterization. It uses visible light to detect small objects. The use of the light microscope allows the viewing of samples at magnifications of up to 1,000X, often with little or no sample preparation. A sample may be viewed as is, while noting properties such as color, transparency and morphology.
Hey! That looks strangely similar to the equation for the energy of a photon. Yup. If you like, you can use light with a frequency of f to induce the transition from one energy level to another. Even better, it doesn’t matter if this transition is from a higher to lower or lower to higher energy level. This oscillating perturbation can explain both absorption AND emission of light.
But wait! There’s more. What if you use Schrodinger’s equation to look at a particle in a one dimensional box? Why would you do this? Because it is mathematically simple and because we can use it to explore some of the results of a quantum system. From Schrodinger’s equation, you would find that the particle can only exist at certain discrete energies. This is really one of the key points of quantum mechanics (it’s the quant in quantum).
Electronmicroscope
Quantum Mechanics. I am going to skip many of the very interesting details, but let me just say that I can use the following model the behavior of a super tiny particle in a box. Here is an older post with most of the particle in a box details. Knock yourself out with that.
But here is the crazy part (I know, you probably think this whole post is crazy): the photoelectric effect can be explained with a classical wave model of light along with a quantum model of matter. Really, it can. Skipping the details, let me just say (and you can look in your quantum mechanics book to verify this) that if you have a particle with energy E1 and you want it to transition to the energy level E2 you can do that by adding a time-varying potential such that:
“Is light a particle or a wave? This is a difficult question - the answer is that in some situations light behaves as a particle and in others it behaves as a wave.”
Introduction toopticalmicroscopy
2. Light as a particle: The textbook might start off with some experimental evidence from the historic photoelectric effect to show that the wave model of light doesn’t always describe what happens.
But then why is the photon model in textbooks? I would say it is because of educational inertia. Who writes the textbooks? If you answer “people”, then you are correct. But where do these “people” learn physics? If you said “textbooks”, that would be a fairly nice answer. So, people learn from textbooks that have photons. Next they write a textbook, so clearly they will have photons in their books. Simple.
PolarizedopticalMicroscopy
Momentum. When you start looking at momentum, it is almost always (except in the awesome textbook Matter and Interactions) defined as:
1. Light as a wave: Light can be described (modeled) as an electromagnetic wave. In this model, a changing electric field creates a changing magnetic field. This changing magnetic field then creates a changing electric field and BOOM - you have light. Unlike many other waves (sound, water waves, waves in a football stadium), light does not need a medium to “wave” in.
“It is high time to give up the use of the word ‘photon’, and of a bad concept which will shortly be a century old. Radiation does not consist of particles and the classical, i.e., non-quantum limit of QTR is described by Maxwell’s Equations for the EM fields, which do not involve particles.”
My favorite quantum analogy is a staircase. For a staircase you can be on one step or the next step but you really can’t be in between steps. In this case, you could say that height is quantized. The same is true for a particle in a box or an electron in a hydrogen atom. There are only certain possible energy levels.
That is still wrong, but better. However, we don’t often use the better model for the gravitational force near the surface of the Earth. Why? Because the mg model works well enough. Also, the two models agree on the surface of the Earth just like the two expressions for the proton momentum agree for “slow” speeds.
Specialized observational and illumination techniques allow the precise measurement of optical properties of transparent materials.
This is great. It’s simple and it’s useful. It goes great with the momentum principle that says that the net force on an object is the time rate of change of momentum. Of course, you could also say it is wrong. What if you have a proton moving at 90 percent the speed of light? In that case, you can’t use this definition of momentum with the momentum principle. Instead, you have to use this model:
What is amicroscope
“All these fifty years of conscious brooding have brought me no nearer to the answer to the question, ‘what are light quanta?’ Nowadays, every Tom, Dick, and Harry thinks he knows it, but he is mistaken.”
This is Schrodinger’s equation and Ψ is called the wave function. It doesn’t give you anything you could directly measure, but from it you could get the probability density - or a description of where a particle is likely to be found (or really, anything else you can know about the particle).
Leicaoptical microscope
Here h is Planck’s constant and λ is the wavelength of the light and f the frequency. With the photon model, a brighter light just produces more photons per second.
Our resource center archives our case studies, published articles, blogs, webinars, and image galleries. Discover ways microscopy has made a meaningful impact.
Hat Tip to David Norwood. Really, it's his fault that I was thinking about this whole issue. However, he did offer some nice suggestions for this post.
Light microscope
That’s nice, right? Some people call this the “relativistic momentum”. However, I like to call this just plain momentum. But what does this have to do with two models for light? Well, what if I wanted to find the momentum of a proton going at just 10% the speed of light? Which model would I use? The answer depends on how quickly you want to calculate this and how accurate you want your answer to be. Yes, I know “quick” is relative.
You have been very patient. I know you want to talk about photons, but I had to get the model stuff out of the way. But like I said, just about every introductory physics textbook talks about photons using the photoelectric effect as a basis for this model.
I don’t know why, but I expect some people to not be so happy with this post. In general, people have one of the following two responses to this kind of argument.
It will then say that we can model light as individual “things” (some books actually say particles and others just say photons). These light “things” have energy that depends on the wavelength such that:
We always have multiple models for things that we see. However, they are different than this wave-particle model of light. Let’s look at a few other models.
Appeal to Authority: I admit that sometimes, things get confusing. In case any of my arguments don’t make any sense, I will add some opinions from experts (meaning people that know more than I do).
How to uselight microscope
It’s in your physics textbook, go look. It says that you can either model light as an electromagnetic wave OR you can model light a stream of photons. You can’t use both models at the same time. It��’s one or the other. It says that, go look.
My main point here is that the photon isn’t what you think it is. It isn’t a tiny little ball of light. It isn’t light as a particle. However, light is still pretty weird. There is a quantum nature to the electric and magnetic fields in light (quantum theory of radiation). But most of the stuff you look at can be explained using a classical wave model of light and a quantized model for matter.
Just to be clear: the quantum model of stuff is just like the other models above. It slowly gives a different result from the classical model of stuff.
Because the sample is not isolated from the microscopist, it can be probed and manipulated in order to judge physical properties such as hardness and elasticity. In microchemical testing, standard qualitative chemical tests are applied in miniaturized format to determine sample composition.
© 2025 Condé Nast. All rights reserved. WIRED may earn a portion of sales from products that are purchased through our site as part of our Affiliate Partnerships with retailers. The material on this site may not be reproduced, distributed, transmitted, cached or otherwise used, except with the prior written permission of Condé Nast. Ad Choices
There is a reason for this. Albert Einstein won the Nobel Prize in 1921 in part for his explanation of the photoelectric effect. Of course, Einstein did some other awesome stuff. In particular, the general and special theory of relativity. But the Nobel Prize didn’t mention this - just the photoelectric effect. However, during Einstein’s acceptance speech for the Nobel Prize, he talked about relativity and not the photoelectric effect.
Perhaps the most recent is this quote from W.E. Lamb, Jr’s paper “Anti-photon” - Lamb Jr, Willis E. "Anti-photon." Applied Physics B 60.2-3 (1995): 77-84.:




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500