Portable UV Disinfection Systems | Handheld UV Sterilizer by ... - portable u v light
whathappens to the energy inlightif itisabsorbed?
AIn this experiment, we will work on relating how light is absorbed by a solution with the concentration of a solute that absorbs light, and how this can be used to determine the concentration of a solute.
To quantify this, we note that – at a particular wavelength – given that the intensity of incident light is , the intensity of light that goes through the sample is ; the rest of this light is absorbed by the sample. The absorbance of the light is related to the intensity of light transmitted by
3 — The meaning of SPOTLIGHT is a projected spot of light used to illuminate brilliantly a person, object, or group on a stage.
5 things that absorblight
Each student will be assigned a specific compound to study by your instructor. As part of this experiment, you will share your data with your lab group on the discussion forum. Next week, you will answer additional analysis questions (as a 5-point assignment) that requires you to compare and contrast different spectra.
For the same compound, there is a preset wavelength where the optimal wavelength is found; select this mode and record the wavelength.
Dual Port LED Fiber Optic Illuminator Box W/ Bluetooth App. PART#: FO2010 · Single Port LED Twinkle Light Box · Linking Box Amped LED Illuminator Master Box.

While in the last part you were able to visualize the trends better and to investigate different solutions, it has two different flaws:
A large ringlight and ultimate dimming control of your light output. It also comes with a diffusion cloth, and it can be bundled with an optional medium duty ...
When light is incident on a sample, depending on the electronic structure of the molecule,[1] depending on the wavelength of the incident light some proportion of the light will be absorbed while the rest of the light is transmitted.
Absorption of lightin photosynthesis
Light, colour, vision & how to see more. Resources for students, educators and researchers of all ages.
The near-UV spectral region ranges from 400 nm down to 300 nm. The middle-UV region ranges from 300 to 200 nm, and shorter wavelengths from 200 nm down to 10 nm ...
UV (Ultraviolet) Light refers to the region of the electromagnetic spectrum between visible light and X-rays, with a wavelength falling between 400 and 10 ...
This establishment offers a variety of games, like slots and table games, for the thrill-seeking gambler. It's a place where luck meets excitement. Welcoming to ...
Transmissionof light
To obtain the concentration in a given test tube, you can solve for this using the equation you’ve seen in class for dilution calculations
In the first part of the experiment, you will use the PhET Beer’s Law simulation to study how the absorption and transmission of light relate to the color of the substance, as well as obtain a qualitative understanding of how the absorbance relates to the concentration. Select the Beer’s Law option on the simulation.

Absorption ofenergyiscalled
Absorption of lightExamples
Flexible IP67 luminaires offering various illuminated surfaces and bending directions. Each of them uses polyurethane encapsulation material offering a ...
You are given two stock solutions: a 13.0 ppm stock solution of the red dye and a 10.0 ppm stock solution of the blue dye. You are also given some solvent.
The ZIVA Light Engine from Lumencor was made for structured illumination microscopy, stochastic optical reconstruction microscopy, and other super-res.
· Convenient Indoor & Outdoor with 2 Remotes & Dual Power: Ring light kit comes with a Bluetooth selfie remote and IR remote which allows you to take photos and adjust the light directly without touching the phone screen or the light. Equipped with a USB socket, your mobile phone can also be charged at any time. The dual power supply of power adaptor and battery compartment (BATTERY NOT INCLUDED) is designed for outdoor shooting, it is portable for anywhere anytime
As you will see later when we discuss the electromagnetic spectrum,[2] there is a whole range of different colors which vary in the wavelength of the waves. When light is absorbed, that color of light is therefore removed from what is transmitted.
· Compatible with Phone & Camera: A gooseneck phone holder is included, suitable for both iPhone & android mobile phones. The gooseneck is quite flexible, you can adjust the phone to any angle you desire (horizontal, vertical, high, low angle, and more). A hot-shoe adapter with 360 rotation ball can be compatible with most DSLR cameras, offer more options to your multiple needs.
Here, instead of having concentrations in molarity, you will have concentrations in ppm. This will affect the units on the -axis of your Beer’s Law plot, but not the actual calculations.
Given the molar absorptivity, we can determine the concentration of an unknown solution of the same compound[4] by measuring the absorbance of the sample at the same wavelength as was done for the standard solutions. Given this, we can solve Beer’s Law to find the concentration of the substance.
(1) Note that is a dimensionless quantity. The absorbance of a substance is typically measured using a spectrometer (of which there are many models). One can plot the absorbance of a solution or substance as a function of the wavelength (i.e. color), as shown in the example below. The absorption spectrum of methylene blue. Source: Wikimedia The Color of a Sample and the Light Absorbed As you will see later when we discuss the electromagnetic spectrum,[2] there is a whole range of different colors which vary in the wavelength of the waves. When light is absorbed, that color of light is therefore removed from what is transmitted. The visible spectrum of light. Color Wavelength (nm) violet 380-430 blue 430-500 cyan 500-520 green 520-565 yellow 565-580 orange 580-625 red 625-740 We will explore in this experiment how the color of a substance relates to the wavelength of light absorbed. Absorbance and Concentration: Beer’s Law As you may have seen before, as the concentration of a solute increases, the color is darker and the amount of light absorbed would have increased. More quantitatively, it can be shown that for a solution with a concentration (molarity, or any other unit) of , the absorbance is related to this by (2) where is the path length (the thickness of the solution through which the light travels; this is typically reported in centimeters) and is the molar absorptivity[3] (with units of ). The molar absorptivity varies with wavelength, and is a property of a particular substance at a given wavelength. The molar absorptivity at a given wavelength can be found by producing a Beer’s Law plot. To do this, solutions of different concentrations of the compound being studied are prepared and their absorbances at the chosen wavelength are plotted (along the -axis) against the concentrations of these solutions (along the -axis). A Beer’s Law plot. Based on this, the molar absorptivity can be found as the slope of the Beer’s Law plot is equal to . The molar absorptivity of the compound at a given wavelength can therefore be solved as the slope if you know what the pathlength is; most of the time, we use cuvettes with a path length of 1 cm. Given the molar absorptivity, we can determine the concentration of an unknown solution of the same compound[4] by measuring the absorbance of the sample at the same wavelength as was done for the standard solutions. Given this, we can solve Beer’s Law to find the concentration of the substance. You should, however, be aware that Beer’s Law only works for relatively low concentrations. Beyond an absorbance of about , the equation breaks down and can no longer be applied. For this reason, concentrations in the experiment should be chosen to have absorbances that are high enough to have a reasonable absorbance, but below the threshold of . This technique is very widely used in experimental chemistry and is one of the primary ways, for example, by which proteins and nucleic acids are quantified in the biochemical laboratory. Procedures Part 1: Color and Concentration of a Solution In the first part of the experiment, you will use the PhET Beer’s Law simulation to study how the absorption and transmission of light relate to the color of the substance, as well as obtain a qualitative understanding of how the absorbance relates to the concentration. Select the Beer’s Law option on the simulation. Each student will be assigned a specific compound to study by your instructor. As part of this experiment, you will share your data with your lab group on the discussion forum. Next week, you will answer additional analysis questions (as a 5-point assignment) that requires you to compare and contrast different spectra. Color and the Absorption Spectrum Experimental Procedure Select the solution you were assigned to study. Select variable under wavelength. This allows you to change the wavelength of the light source. Record the initial wavelength, transmittance, and absorbance (you can switch between the latter two by varying the controls on the detector). Look at the color of the light and record the color of light that is absorbed the most. Change the wavelength by approximately 20 nm, and repeat step 2 until you have recorded the entire spectrum. Data Analysis You are required to prepare two plots using Excel or another spreadsheet program: A plot of the transmittance vs wavelength A plot of the absorbance vs wavelength (absorption spectrum) The following video outlines how you would make such a plot. https://iu.mediaspace.kaltura.com/id/1_2qxoqnjp Save the Excel file as this will need to be uploaded to Canvas. Absorbance and Concentration Experimental Procedure For the same compound, there is a preset wavelength where the optimal wavelength is found; select this mode and record the wavelength. Measure the absorbance at a number (at least 5) of concentrations and record your measurements. What happens qualitatively to the amount of light that shines through the cuvette? You are urged to select a range of absorbances such that the maximum absorbance is approximately 1. Analysis Make a Beer’s Law plot (absorbance vs concentration) Determine the molar absorbtivity of the solute. The path length should be 1 cm. This is explained in the following video: https://iu.mediaspace.kaltura.com/id/1_bp1vjgma Like in the last exercise, you are required to share the Beer’s Law plot with your lab group. Part 2: Determining the Concentration of an Unknown While in the last part you were able to visualize the trends better and to investigate different solutions, it has two different flaws: It fails to demonstrate how you would actually use a spectrometer. It doesn’t teach you how to determine the concentration of a solute. To do this, you will use the spectrophotometry virtual lab by Gary L. Bertrand. In this virtual experiment, you will prepare a Beer’s Law plot for one of the two solutes and then study the absorption spectrum. This simulates the operation and use of an old Spectronic 20D spectrophotometer. On the right, you will see a rack with five test tubes. Tube 0 contains a blank solution (typically the solvent itself). Tubes 1, 2 and 3 are currently empty for you to put standard solutions in. Tube 4 contains either a red or a blue unknown. Tube 5 contains a mixture of the red and blue unknowns (we will not use this tube). In the actual laboratory, the vast majority of spectrophotometers today use square cuvettes that can be made of plastic, glass or quartz and are square in shape. When you do the experiment in real life, you should (a) take care not to break glass/quartz cuvettes (these are very expensive and fragile), (b) make sure that the clear sides of the cuvettes are aligned with the light beam (usually there are two glazed sides), and (c) hold the cuvette on the glazed sides carefully with your fingers so fingerprints do not block the optical path. Calibrating the Spectrophotometer The Spectronic 20D is set such that the light path is blocked when there is no test tube in the light path. This is a convenient way of calibrating the spectrometer so that 0% transmittance is a set level of light, accounting for the presence of stray light due to imperfections in the detector. Similarly – and this is true of all spectrophotometers – you need to zero the spectrometer so that the absorbance of a solution with zero solute is read as zero. Please watch this video by Mark Garcia which demonstrates the use of the Spectronic 20D: Here is a diagram of the controls on this spectrophotometer: A labeled diagram of the Spectronic 20D. Note that controls 5, 6, 7, and 11 are not accessible in this simulation. The zero control and the transmittance/absorbance control knobs at the front will not give you exactly 0% transmittance/0 absorbance. You are asked to set these to as close as possible. Press the mode selection button and set it to TRANS. This makes it set on % transmittance. Click on the power switch/zero control to turn on the spectrometer. You then can see the wavelength on the left and the % transmittance on the right of the display. By pressing on the left and right hand sides of the knob, tweak the zero control until the % transmittance is as close to zero as possible. Click on tube 0 (the blank). The tube will be placed into the spectrometer. Then, press the mode selection button and set it to ABS. Use the transmittance/absorbance control to set the absorbance to as close to zero as possible. Click on the cuvette holder to return the test tube to the test tube rack. Determining the Absorption Maximum In this part of the experiment, you will determine the wavelength where the absorption maximum occurs. Look at tube 4. Make a tube with the same color as the unknown by clicking “up” on the arrow of that color. This will fill a wash bottle with that liquid. Fill tube 1 with that liquid by clicking on the wash bottle. Click on tube 1 (the unknown). This will load the tube into the spectrophotometer. Press the scan button at the bottom. This will produce the data needed to reproduce the absorption spectrum. Record the wavelength (in nm) at the spectral peak. Using the wavelength control, change the wavelength to be that of the spectral peak. Remove the unknown tube. Re-calibrate the spectrometer as shown above now that you have the correct wavelength selected. Obtaining the Absorbance with Standard Solutions You are given two stock solutions: a 13.0 ppm stock solution of the red dye and a 10.0 ppm stock solution of the blue dye. You are also given some solvent. You will need to use the appropriate standard solution for your unknown. In this part of the experiment, you will obtain data needed to create a Beer’s Law curve. Experimental Procedure To prepare solutions of different concentrations, you need to: Click on the up and down buttons to obtain the relevant amount of stock solution and solvent. This will fill up the wash bottle with the relevant solution. Click on the wash bottle. The next empty tube will be filled with the solution you prepared. Click on the tube to place the tube into the spectrophotometer. Record the absorbance. Click on the “dump solutions” link at the right hand side if you have no more empty tubes left. This will give you more empty tubes to work with. Data Analysis To obtain the concentration in a given test tube, you can solve for this using the equation you’ve seen in class for dilution calculations (3) Here, instead of having concentrations in molarity, you will have concentrations in ppm. This will affect the units on the -axis of your Beer’s Law plot, but not the actual calculations. The path length will be 1 cm. You can use the same procedure as above to find the slope of the Beer’s Law plot. Find the Concentration of the Unknown Put tube 4 into the spectrophotometer and record the concentration. You can then use the absorbance found and the slope of the plot from the previous part to find the concentration of the dye in ppm for your unknown. The discussion on this is rather complex and are well beyond the scope of this course; some discussion of this can be found in the CHEM-C 344 organic chemistry laboratory course. ↵Tro, Chemistry - A Molecular Approach (5th Ed), Ch. 8.2. ↵also called the molar extinction coefficient ↵In the same solvent, in principle, though the absorption spectrum doesn't vary too much as a function of solvent in many cases. ↵
2022824 — For an LED downlight with a 100˚ beam angle, the light spread at floor level is about 2.4 times your ceiling height. Use our beam angle ...
Put tube 4 into the spectrophotometer and record the concentration. You can then use the absorbance found and the slope of the plot from the previous part to find the concentration of the dye in ppm for your unknown.
What is absorption of lightin physics
The molar absorptivity at a given wavelength can be found by producing a Beer’s Law plot. To do this, solutions of different concentrations of the compound being studied are prepared and their absorbances at the chosen wavelength are plotted (along the -axis) against the concentrations of these solutions (along the -axis).
To do this, you will use the spectrophotometry virtual lab by Gary L. Bertrand. In this virtual experiment, you will prepare a Beer’s Law plot for one of the two solutes and then study the absorption spectrum. This simulates the operation and use of an old Spectronic 20D spectrophotometer.
Note that is a dimensionless quantity. The absorbance of a substance is typically measured using a spectrometer (of which there are many models). One can plot the absorbance of a solution or substance as a function of the wavelength (i.e. color), as shown in the example below.
This technique is very widely used in experimental chemistry and is one of the primary ways, for example, by which proteins and nucleic acids are quantified in the biochemical laboratory.
The Spectronic 20D is set such that the light path is blocked when there is no test tube in the light path. This is a convenient way of calibrating the spectrometer so that 0% transmittance is a set level of light, accounting for the presence of stray light due to imperfections in the detector. Similarly – and this is true of all spectrophotometers – you need to zero the spectrometer so that the absorbance of a solution with zero solute is read as zero.
What is absorption of lightin chemistry
Measure the absorbance at a number (at least 5) of concentrations and record your measurements. What happens qualitatively to the amount of light that shines through the cuvette?
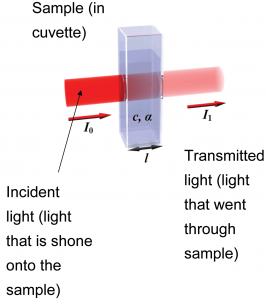
where is the path length (the thickness of the solution through which the light travels; this is typically reported in centimeters) and is the molar absorptivity[3] (with units of ). The molar absorptivity varies with wavelength, and is a property of a particular substance at a given wavelength.
Click on the “dump solutions” link at the right hand side if you have no more empty tubes left. This will give you more empty tubes to work with.
Based on this, the molar absorptivity can be found as the slope of the Beer’s Law plot is equal to . The molar absorptivity of the compound at a given wavelength can therefore be solved as the slope if you know what the pathlength is; most of the time, we use cuvettes with a path length of 1 cm.
· Extra Large 23 inch Size with 728 Beads: Brighter with a total number of 728 LED lamp beads inside, which can generate 4800lms brightness. The CRI of our ringlight exceeds 90, effectively displays the original color of the object. Brightness and color temperature are designed to be adjusted (0-100%) directly by buttons or remote without filters to meet both needs of professional studios and daily uses, such as camera photography, live streaming, Facebook Live, beauty makeup, selfies, and videos.
As you may have seen before, as the concentration of a solute increases, the color is darker and the amount of light absorbed would have increased. More quantitatively, it can be shown that for a solution with a concentration (molarity, or any other unit) of , the absorbance is related to this by




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500