Magnifying Glasses with LED Light, up to 25x magnification - large magnifying glass with light
The data obtained from this study were summarized, and descriptive statistics were tabulated as mean ± standard deviation and median and minimum-maximum for continuous variables depending on their distribution. Categorical variables were summarized as numbers and percentages. The normality test of numerical variables was verified by means of the Kolmogorov-Smirnov and Shapiro-Wilk tests. The paired t-test was used to examine whether the roughness value changed before and after the procedure. In comparisons of more than two independent groups, the Kruskal-Wallis H test was used when numerical variables did not show normal distribution. Differences among the groups in non-parametric tests were evaluated by the Dwass-Steel-Critchlow-Fligner test. Statistical analyses were performed using the IBM SPSS Statistics for Windows (version 20.0) program, and the significance level was defined as 0.05 (p-value).
In spectroscopy, the frequency of the electromagnetic radiation being used is usually expressed in hertz (Hz), that is, cycles per second. Note that 1 Hz = s−1.
• Since color stability is an important factor in the success of treatment in aesthetic composite restorations, the stability of this novel smart monochromatic RBC material against discoloration is a matter of interest.
The notion that electromagnetic radiation contains a quantifiable amount of energy can perhaps be better understood if we talk about light as a stream of particles, called photons, rather than as a wave. (Recall the concept known as ‘wave-particle duality’: at the quantum level, wave behavior and particle behavior become indistinguishable, and very small particles have an observable ‘wavelength’). If we describe light as a stream of photons, the energy of a particular wavelength can be expressed as:
Note: You should not try to memorize the relationship between energy and wavelength in the form in which it is given here. Instead, you should be prepared to work from first principles using:
Provides 99% protection from harmful UV-A rays. Impact resistant 100% polycarbonate lens provides excellent eyes protection.
Color stability and surface texture are the most important characteristics of esthetic restorative materials aiming to provide a personalized smile.1 The maintenance of color throughout the functional lifetime of restorations plays a significant role in the durability of the treatment. However, this characteristic is not constant among dental materials.2,3 Various inorganic particles with different components, such as silica, alumina, zirconia, silicate glass, quartz, and ceramics, have been employed in the resin matrix of dental resin-based composites (RBC) as reinforcing filler phases. In addition to the composition of fillers, other properties, such as the filler particle content, size, size distribution, shape, morphology, porosity, and surface characteristics, play a critical role in the development of dental RBCs for specific applications and purposes.4
After the initial color measurements were taken and recorded, the samples (n=10) were kept in four different types of beverages (distilled water, coffee, cola, and orange juice) at 37 °C for one week. The beverages were renewed daily, the samples were kept in the beverages throughout the day, and final measurements were taken and recorded at the end of one week. For standardization, the same immersion period was applied in all beverages.
The Minolta Chromascop colorimeter device (Chroma Me-ter CR 321, Minolta, Osaka, Japan) was used to evaluate color changes. It detects the reflected colors of surfaces using compact tristimulus color analysis. The measurement area was 3 mm and had 45° environmental illumination, and the viewing angle was 0°. Measurements are given as L* a* b*. During the measurements of the samples, the calibration of the instrument was checked before each color measurement step by using a standard white background. Measurements were repeated three times for each sample, and the average of the values was determined. The amount of color change is expressed as ΔE and calculated as follows;
where E is energy in kcal/mol, λ (the Greek letter lambda) is wavelength in meters, c is 3.00 x 108 m/s (the speed of light), and h is 9.537 x 10-14 kcal•s•mol-1, a number known as Planck’s constant.
In general, RBCs with low filler contents are known to exhibit more color change.35,36 In this study, when the weight ratios of the particles in their content were examined, zenit (83%) had the highest value, followed by harmonize (81%) and OM (79%) (Table 1). However, in regard to the average ∆E values (p=0.35), the particle weight was not in line with the results of this study, and the results were not statistically significant (Table 5, 6). We examined the monomer structures of the RBCs as we believed that the close similarity in the weight percentages of the RBCs used in this study contributed to this result.
By observing which wavelengths a molecule absorbs, and to what extent it absorbs them, we can gain information about the nature of the energetic transitions that a molecule is able to undergo, and thus information about its structure.
However, the choice of color increases the time spent in the dental unit and makes the color selection process subjective. The use of smart technologies in the production of RBCs has recently been applied in order to eliminate all these disadvantages. Manufacturers have developed a resin-based OM composite, which has been formulated based on the concept of “Wide Color Matching.” OM creates shades which can cover a large number of natural tooth shades in order to reduce the time required for shade selection and also the number of composite shades needed in stock. It has been claimed that this new dental composite produced with Smart Chromatic Technology can match the tooth color thanks to its optical structure whereby the particles reflect the color of the surrounding tooth structure.14,15 Therefore, in the current study, a novel single shade composite resin with spherical fillers was compared with the nanohybrid composite harmonize, which imparts a chameleon effect, and the zenit composite containing fine radiopaque porcelain fillers.
Studies reporting that coffee causes more discoloration than other beverages support this result.26-28 Bagheri et al.29 argued that although cola harms the surface integrity of composite materials due to its low pH value, it does not cause discoloration as much as coffee and tea due to the absence of yellow dye material. Sirin Karaarslan et al.30 reported that there was a decrease in L-values in all of the samples after the aging process, and a decrease in L-value indicates that the samples darkened. In the present study, the ΔL* values decreased significantly as expected in all composite groups exposed to coffee, cola, and orange juice beverages (Table 4). ΔL* results are consistent with many studies examining color changes in composite resins exposed to different beverages. In these studies, the effect of discoloration has been observed to result in negative ΔL* values for composite materials.31-33 In the CIE Lab system, it is stated that the b* coordinate is associated with yellow and blue color. A positive b* value indicates the amount of yellow, and a negative b* value indicates the amount of blue. Tekçe et al.31 found that in all composite resins, there was a shift towards the blue direction (-Δb*) with distilled water exposure, and towards the yellow direction (+Δb*) with black tea exposure. Poggio et al.32 also found a significant increase in the Δb values of composite resins exposed to coffee, and their findings support our study. In another study, it was reported that yellow discolorants in coffee cause discoloration by showing low polarity, thus adhering to the surface and penetrating deeply.34 In the present study, the positive Δb value in all samples immersed in the coffee and orange beverages was thought to be related to this situation (Table 5), with the highest Δb value belonging to the zenit composite (Δb=5.81). Although all staining beverages resulted in an increase in the Δb value, we noted that zenit exhibited the highest level of Δb value among the groups, indicating that the effects of the orange solution on zenit were greater compared to the other RBCs.
One of the most important factors affecting the degree of discoloration of RBC is the type of monomer.37 RBCs with high triethylene glycol dimethacrylate (TEGDMA) content are more susceptible to discoloration than RBCs containing urethane dimethacrylate (UDMA) because UDMA is a more color-resistant monomer. This is due to the low water absorption of UDMA monomer and the sufficient degree of visible light polymerization.28,38,39 When the average values in the present study were taken into account, the OM resin material containing UDMA and TEGDMA had the lowest ΔE value (3.33), however, no statistically significant difference was observed between the ΔE values of the RBC materials (Table 5). The highest ΔE value was obtained in the orange juice solution in zenit (ΔE=7.02) (Table 3). In parallel with this result, a study by Gregor et al.40 found that the nanoceramic-based Ceram.X Duo was highly affected by acidic fruit juice, and this might be due to a possible acidic attack by the polysiloxane components of the fruit juice. This explains why the nanoceramic-based zenit composite containing butanediol dimethoxylated and glass particles had the highest value.
Inline Lighting (@inlinelighting) on TikTok | 2067 Likes. 205 Followers. Premier Lighting Showrooms Furniture, Decor, Art, & More✨ Serving the Southeast.
When talking about electromagnetic waves, we can refer either to wavelength or to frequency – the two values are interconverted using the simple expression:
Visible light has a wavelength range of about 400-700 nm. What is the corresponding frequency range? What is the corresponding energy range, in kcal/mol of photons?
From your studies in general chemistry or physics, you should be familiar with the idea that electromagnetic radiation is a form of energy that possesses wave character and travels through space at a speed of 3.00 × 108m · s−1. However, such radiation also displays some of the properties of particles, and on occasion it is more convenient to think of electromagnetic radiation as consisting of a stream of particles called photons.
Illuminate your adventures with handheld outdoor spotlights & LED spotlights from Bass Pro Shops. Shop reliable lighting on the go. Free Shipping on $50.
• “Omnichroma” is a monochrome composite with 260 nm spherical filler, produced with smart chromatic technology, and the ability to adapt to the color of the applied tooth.
In regard to the staining beverages, when the roughness of each composite filling material was evaluated before staining, there was no significant difference among the harmonize, OM, and zenit groups (p=0.738, p=0.969, and p=0.940, respectively) (Figure 1, 2, 3). Similarly, no statistically significant difference was observed among the post-treatment roughness of each composite resin (p=0.843, p=0.229, and p=0.745, respectively). A significant increase in roughness was observed in harmonize immersed in the coffee solution (p=0.024) and OM immersed in the cola solution and water (p=0.021, p=0.038, respectively) (Table 2).
E = hv, where h = Plank’s constant = 6.626 × 10−34J · s. c = λv, where c = the speed of light = 3.00 × 108m · s−1. Avogadro’s number = 6.02 × 1023 mol−1
Lights derive settings for their illumination color, shadow color and how much they contribute to diffuse and specular shading from the light material item.
Since composite restorations have different shades and technical sensitivities, success is highly dependent on the skill of the dentist and the choice of shade. This complex process causes the physician to employ a trial-and-error method and increases the time spent in the dental chair. Therefore, the researchers aimed to simplify and reduce the number of shadows based on color interactions. The chameleon or blending effect is the ability of dental materials to adapt to the color of the surrounding dental hard tissue so that color mismatches are compensated to some extent.5 These materials, which have the properties of imitating natural tooth structures such as enamel and dentine, support the remaining tooth structure. They employ “smart chromatic technology”. However, they can be changed by factors such as temperature, humidity, pH, and stress.6 RBCs have undergone many changes over the years in order to provide positive properties. For this purpose, many composite resin types are available on the market with changes in both their monomers forming the organic polymer matrix phase and their inorganic filler particles.4
• Good color matching eliminates the need for color selection and minimizes in-seat time and wastage of unused composite hues.
This study aimed to assess the color stability and surface roughness of a newly developed single-shade resin-based composite (RBC) utilizing smart chromatic technology in comparison to nanohybrid and nanoceramic RBCs.
Wall Light Silhouette Round ... The PROLED WALL LIGHT SILHOUETTE series is designed for wall mounting and offers various design possibilities in architectural ...
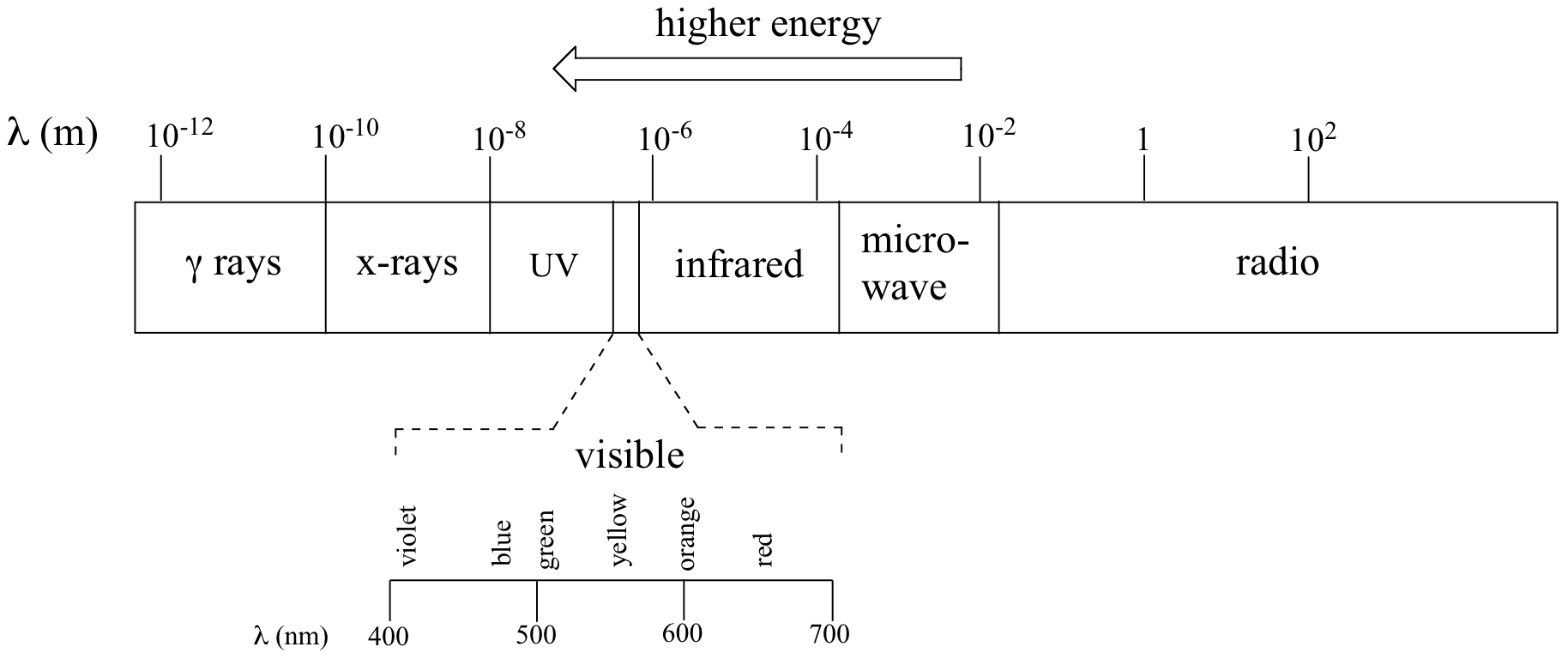
Just like ocean waves, electromagnetic waves travel in a defined direction. While the speed of ocean waves can vary, however, the speed of electromagnetic waves – commonly referred to as the speed of light – is essentially a constant, approximately 300 million meters per second. This is true whether we are talking about gamma radiation or visible light. Obviously, there is a big difference between these two types of waves – we are surrounded by the latter for more than half of our time on earth, whereas we hopefully never become exposed to the former to any significant degree. The different properties of the various types of electromagnetic radiation are due to differences in their wavelengths, and the corresponding differences in their energies: shorter wavelengths correspond to higher energy.
The overall color change (∆E*) mean values of the RBCs for all staining beverages are shown in Table 3. When the RBCs were compared with each other in regard to the color change in distilled water, coffee, and cola solutions, there was no significant difference between the ΔE* values. The color change of harmonize and OM composite resins after immersion in distilled water, orange juice, and cola was found to be significantly lower than the ΔE* value obtained after immersion in coffee (p<0.001). When the color stability of the RBCs was compared after immersion in the orange juice beverage, zenit showed the highest ΔE* value (6.47±1.65), and this color change was considered significant when compared to harmonize and OM (Table 3). Among all RBCs, the ΔE* value of the distilled water was the lowest (1.26), while the ΔE* value of the coffee was the highest (7.04), and this difference was determined to be statistically significant (p<0.001) (Table 4). The differences among the ΔL, Δa, and Δb color change medians of the composite materials according to the staining beverages used were statistically significant (p=0.034, p<0.001, and p=0.001, respectively), and these differences were examined through multiple comparisons (Table 5, 6). When we examined the color change values (ΔE) according to the staining beverages used, the lowest value was found in the distilled water (ΔE=1.26) and the highest degree of color change was found in the coffee group (ΔE=7.04) (Table 4, Figure 4).
These generalized ideas may all sound quite confusing at this point, but things will become much clearer as we begin to discuss specific examples.
High-energy radiation (such as gamma- and x-rays) is composed of very short waves – as short as 10-16 meter from crest to crest. Longer waves are far less energetic, and thus are less dangerous to living things. Visible light waves are in the range of 400 – 700 nm (nanometers, or 10-9 m), while radio waves can be several hundred meters in length.
Here is the key to molecular spectroscopy: a given molecule will specifically absorb only those wavelengths which have energies that correspond to the energy difference of the transition that is occurring. Thus, if the transition involves the molecule jumping from ground state A to excited state B, with an energy difference of ΔE, the molecule will specifically absorb radiation with wavelength that corresponds to ΔE, while allowing other wavelengths to pass through unabsorbed.
A total of 120 specimens of RBC discs were prepared on a round metal mold (diameter 6 mm, thickness 2 mm). Distilled water was used as a control. Three different RBCs; harmonize (n=40), omnichroma (OM) (n=40), and zenit (n=40), were immersed into three staining beverages, namely orange juice, cola, and coffee, respectively, as the test groups. Prior to the immersion, the initial roughness and color values were recorded using a profilometer and CIE L* a* b*, respectively. The color changes and surface roughness values were determined again after one week. The normality test of numerical variables were analysed by means of Kolmogorov-Smirnov and Shapiro-Wilk tests (p≤0.05).
High Power LED Lighting HLDL3 Series. Bar lights for long-distance and wide-area irradiation. Suitable for various applications such as ...
In vitro studies can’t fully mimic oral conditions. Clinical factors like nutritional habits, temperature, humidity, microorganisms, oral structure, saliva quantity, and tongue-cheek function are challenging to assess. Discoloration from oral beverages may be diluted by saliva. Changes in temperature and pH levels can impact dental material color and composite restoration properties. Insufficient literature exists comparing smart monochromatic resins, limiting discussions on color changes and roughness values. Additional in vitro and clinical studies are needed to support.
Because electromagnetic radiation travels at a constant speed, each wavelength corresponds to a given frequency, which is the number of times per second that a crest passes a given point. Longer waves have lower frequencies, and shorter waves have higher frequencies. Frequency is commonly reported in hertz (Hz), meaning ‘cycles per second’, or ‘waves per second’. The standard unit for frequency is s-1.
Recently, a newly developed monochrome RBC [omnichroma; (OM), Tokuyama Dental, Tokyo, Japan] was introduced to shorten treatment time and reduce the clinician’s difficulty in matching colors. The manufacturer claims that the structural color in the OM can mimic the color of the surrounding tooth, regardless of its shade.7 Homogenized spherical filler particles with a size of 260 nm were used via the “sol-gel method” to obtain uniform filler particles with reflectivity. OM fillers change the light transmitted through the red-yellow area of the color spectrum, allowing it to match the color of the patient’s neighboring teeth. OM’s wide color matching ability minimizes the waste of unused composite hues by reducing the time spent on color selection and the time spent in the chair by the patient.7,8 However, to date, there has not been enough research on the physical properties and color adjustment of these composite resins produced with this technology.
Electromagnetic radiation, as you may recall from a previous chemistry or physics class, is composed of electrical and magnetic waves which oscillate on perpendicular planes. Visible light is electromagnetic radiation. So are the gamma rays that are emitted by spent nuclear fuel, the x-rays that a doctor uses to visualize your bones, the ultraviolet light that causes a painful sunburn when you forget to apply sun block, the infrared light that the army uses in night-vision goggles, the microwaves that you use to heat up your frozen burritos, and the radio-frequency waves that bring music to anybody who is old-fashioned enough to still listen to FM or AM radio.
This study aimed to evaluate the surface roughness and color stability of OM, a newly developed single-shade composite resin containing spherical particles, by comparing it with nanohybrid and nanoceramic RBCs. The null hypothesis of the present study was that the surface roughness and color change of smart chromatic composite resins immersed in different beverages (distilled water, coffee, cola, and orange juice) were not significantly different from certain multi-shade RBCs with various contents.
ML Landsman · 1976 · 1022 — The absorption spectrum of indocyanine green depends on the nature of the solvent medium and on the dye concentration. Binding to plasma proteins causes the ...
There was no significant difference between the initial surface roughness values (Ra) among the groups. However, a significant increase was observed in the roughness values of harmonize immersed in coffee (p=0.024) and OM immersed in cola (p=0.021). The color stability of RBC was significantly affected by the immersion period, and coffee caused the highest discoloration (p<0.001).
Notice in the figure above that visible light takes up just a narrow band of the full spectrum. White light from the sun or a light bulb is a mixture of all of the visible wavelengths. You see the visible region of the electromagnetic spectrum divided into its different wavelengths every time you see a rainbow: violet light has the shortest wavelength, and red light has the longest.
SEKONIC L-308X FLASHMATE LIGHT METER. Sku: 4962294012007. SEKONIC L-308X FLASHMATE LIGHT METER. KD 69.95. Out of stock. Pre Order. Compare. SEKONIC LITEMASTER ...
All RBCs are prone to acceptable surface roughness after staining, and the most stable roughness values belonged to the zenit composite. Coffee had the highest adverse effect on restoration color and caused unacceptable discoloration in all three RBCs (ΔE>3.3). The universal shade RBC OM showed acceptable values in terms of color stability and roughness and may be clinically recommended with a single-color option.
A total of 120 samples were obtained in this study, with 40 samples for each RBC. A cylindrical metal mold of diameter 6 mm, thickness 2 mm was used for composite samples. Mylar strip (Kerr Corp. Orange CA, USA) and cement glass were placed on it in order to obtain a smooth surface. Based on the manufacturer’s instructions, the light emitting diode light device Monitex Bluex, GT1200, (Monitex Industrial Co., Taiwan) was applied for 20 seconds in full power mode, M1 mode, at 1,200 mW/cm2, with a glass coverslip and light device in contact. Following the polymerization process, the samples were kept in distilled water at 37 °C for 24 hours. They were then polished under a water spray (new discs were used for every five samples) by applying a unidirectional rotation motion with light pressure from coarse to fine grain with 4-stage OptiDisc (Kerr Corp. Orange CA, USA) containing aluminum oxide abrasive. The composite resin materials used in this study are listed in Table 1.
In a spectroscopy experiment, electromagnetic radiation of a specified range of wavelengths is allowed to pass through a sample containing a compound of interest. The sample molecules absorb energy from some of the wavelengths, and as a result jump from a low energy ‘ground state’ to some higher energy ‘excited state’. Other wavelengths are not absorbed by the sample molecule, so they pass on through. A detector on the other side of the sample records which wavelengths were absorbed, and to what extent they were absorbed.
where ν (the Greek letter ‘nu’) is frequency in s-1. Visible red light with a wavelength of 700 nm, for example, has a frequency of 4.29 x 1014 Hz, and an energy of 40.9 kcal per mole of photons. The full range of electromagnetic radiation wavelengths is referred to as the electromagnetic spectrum.
Various factors, such as plaque accumulation, the effect of coloring foods and beverages on the composite resin structure, insufficient polymerization of materials, degree of absorption, smoking, frequency of interaction with chemical agents, and the surface roughness of the restoration can cause color changes.11 The roughness and color stability of the composite resin are among the most important factors for a successful esthetic restoration.12 Choosing the right shade is an important esthetic factor in the direct restoration of teeth using RBCs. For the restoration of teeth with different shades, dental material manufacturers have produced a variety of RBCs with different shades and/or translucency. One of the most important problems faced by dentists is the incompatibility of the color between the RBCs used and the natural tooth. In order to eliminate this issue, many factors, such as symmetry, color, translucency, and the surface features need to be considered.13
In this study, the initial roughness measurements were made with the Perthometer M2 (Mahr, Gottingen, Germany) profilometer device. Afterwards, the samples were kept in four different beverages (distilled water, coffee, cola, and orange juice) in an oven at 37 °C for one week, and the beverages were renewed every other day. At the end of this staining process, the final roughness measurements were evaluated. Three measurements were made in the center of the samples with the same device, and Ra values were recorded by calculating the average of these values.9,10 A calibration process was performed after every five measurements.
In many studies16-18, the average critical value was specified as being 0.2 µm for surface roughness. However, there is no accepted threshold value for the assessment of surface roughness to date. In a clinical study conducted by Jones et al.19, patients were able to realize when the mean surface roughness was 0.3 μm. In the present study, a significant increase in the mean roughness values of all composite resins was shown after staining (Figure 1, 2, 3). We think that this may be related to the acidity of the beverages used and the surface irregularities which occur during the finishing process. With regard to the staining beverages used, when the roughness of each composite filling material was evaluated before the treatment, no statistically significant difference was found among the harmonize, OM, and zenit groups (p=0.738, p=0.969, and p=0.940, respectively) (Table 2). An increase in roughness was observed after staining the harmonize composite in coffee and the OM composite in cola. This change might cause corrosion on the composite resin surface due to the phosphoric acid and sugars in the structure of the solution.20,21 de Gouvea et al.21 and Isabel et al.22 determined that different long-chain organic acids in coffee can dissolve and etch restorative materials, thereby causing surface roughness in composite resins. Therefore, the surface roughness of the harmonize composite in the present study supports these results. Also, these differences can be explained by the differences in the polymer matrices, the types of fillers, and the connections between the filler and the polymer matrix.23,24 It was observed that the roughness values of the OM composite material were significantly different (p=0.021) after immersion in cola, but there was no significant difference with harmonize and zenit (p=0.414 and p=0.540, respectively) (Table 2). This difference might be related to the supra nano spherical fillers of the OM composite resin. At the same time, since OM composite resin is a new product, the lack of studies on both its mechanical and esthetic performance limits the interpretation of the success of this material.
The new generation monochrome smart composite resin with spherical filler (OM) is clinically recommended as it shows acceptable values in terms of color stability and roughness. Therefore, OM may be preferred as an alternative to multi-shade composites as it simplifies color selection.
When the RBC materials were evaluated with one another, the differences between the ΔE color changes were not found to be statistically significant (p=0.350) (Table 5). However, a statistically significant difference was found in the evaluation of color changes of OM composite materials according to the different beverages (p<0.001) (Table 6). These results are in line with those of Ebaya et al.5 who concluded that universal shade composites have a satisfactory color and accept surface roughness. In contrast, de Abreu et al.41 and Iyer et al.42 reported that the color adjustment of the monochrome composite was lower than that of the multi-shade composite, and this may cause esthetic problems. Based on all these findings, the null hypothesis of the present study was partially rejected.
A quantum is a small, definite quantity of electromagnetic radiation whose energy is directly proportional to its frequency. (The plural is “quanta.”) If you wish, you can read about the properties of electromagnetic radiation and the relationships among wavelength, frequency and energy, or refer to your general chemistry textbook if you still have it.
Coherent light is the electromagnetic radiation that exhibits synchronization in both the spatial and temporal domains.
In the present study, differences in ΔE values were detected in all samples after they were immersed in the beverages (Table 3). The composite resins tested in the present study showed a significant color change after immersion in coffee and orange juice but not in distilled water or cola (Figure 4). The highest ΔE value was obtained with coffee (ΔE=7.04), followed by orange juice (ΔE=4.11), cola (ΔE=1.84), and distilled water (ΔE=1.26) (Table 4). Ardu et al.25 reported that the amount of discoloration of RBCs varies according to the brand and content of the composite resin; red wine causes the greatest color change among the materials used as staining beverages, followed by coffee, tea, orange juice, and cola.
• “Monochrome composite” is used to denote composite material produced with smart chromatic technology, thus providing a color match for each tooth color.
Listen to ABERRATION OF LIGHT on Spotify · Compilation · Various Artists · 2022 · 14 songs.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500