Light Meters - Grainger Industrial Supply - lighting meter

Whatcompounds absorb UVlight
Lotus 2" Mini Recessed LED Downlights · Lotus 6 Watt 2" Mini LED Round Recessed Downlight - 3000K - 480 Lumens - 24V - White · Lotus 6 Watt 2" Mini LED Round ...
The science of spectroscopy is quite sophisticated. From spectral lines astronomers can determine not only the element, but the temperature and density of that element in the star. The spectral line also can tell us about any magnetic field of the star. The width of the line can tell us how fast the material is moving. We can learn about winds in stars from this. If the lines shift back and forth we can learn that the star may be orbiting another star. We can estimate the mass and size of the star from this. If the lines grow and fade in strength we can learn about the physical changes in the star. Spectral information can also tell us about material around stars. This material may be falling onto the star from a doughnut-shaped disk around the star called an accretion disk. These disks often form around a neutron star or black hole. The light from the stuff between the stars allows astronomers to study the interstellar medium (ISM). This tells us what type of stuff fills the space between the stars. Space is not empty! There is lots of gas and dust between the stars. Spectroscopy is one of the fundamental tools which scientists use to study the Universe. Use Hera to analyze spectra. Updated: August 2013
Spectroscopy can be very useful in helping scientists understand how an object like a black hole, neutron star, or active galaxy produces light, how fast it is moving, and what elements it is composed of. Spectra can be produced for any energy of light, from low-energy radio waves to very high-energy gamma rays.
A service of the High Energy Astrophysics Science Archive Research Center (HEASARC), Dr. Andy Ptak (Director), within the Astrophysics Science Division (ASD) at NASA/GSFC
Each element in the periodic table can appear in gaseous form and will produce a series of bright lines unique to that element. Hydrogen will not look like helium which will not look like carbon which will not look like iron... and so on. Thus, astronomers can identify what kinds of stuff are in stars from the lines they find in the star's spectrum. This type of study is called spectroscopy. The science of spectroscopy is quite sophisticated. From spectral lines astronomers can determine not only the element, but the temperature and density of that element in the star. The spectral line also can tell us about any magnetic field of the star. The width of the line can tell us how fast the material is moving. We can learn about winds in stars from this. If the lines shift back and forth we can learn that the star may be orbiting another star. We can estimate the mass and size of the star from this. If the lines grow and fade in strength we can learn about the physical changes in the star. Spectral information can also tell us about material around stars. This material may be falling onto the star from a doughnut-shaped disk around the star called an accretion disk. These disks often form around a neutron star or black hole. The light from the stuff between the stars allows astronomers to study the interstellar medium (ISM). This tells us what type of stuff fills the space between the stars. Space is not empty! There is lots of gas and dust between the stars. Spectroscopy is one of the fundamental tools which scientists use to study the Universe. Use Hera to analyze spectra. Updated: August 2013
Each spectrum holds a wide variety of information. For instance, there are many different mechanisms by which an object, like a star, can produce light. Each of these mechanisms has a characteristic spectrum.
What absorbs lightin photosynthesis
A-Level Chemistry is a one-stop site for all your Chemistry Teaching and Revision needs. We offer detailed revision materials for students, and teaching resources for teachers and tutors.
Whatsubstanceabsorbs lightenergy during photosynthesis
Find LED spotlights at Lowe's today. Shop LED flood lights and a variety of lighting & ceiling fans products online at Lowes.com.
Light absorption is the process in which light is absorbed by matter and converted into energy. In an atom, electrons vibrate at a specific frequency – this is called the natural frequency. If a wave of light hits a material in which the electrons are vibrating at the same frequency as the wave of light, the electrons will absorb the energy and convert it into vibrational motion. This is why objects have different colours – different materials’ electrons will vibrate at different rates, and therefore absorb different frequencies of light.
Marine LED lighting solutions engineered for interior and exterior sea vessels, yachts, heavy-duty equipment, commercial fishing, facilities, boats, ...
What absorbs lightin plants
Emission spectroscopy is used to measure the photons released when an electron falls to a lower energy level after becoming excited. The emission spectrum of a certain material is shown by a black band with separated coloured lines. These colours lines are the parts of the spectrum where photons have been released from the electrons when they fall to a lower energy level.
20241123 — While high magnification without high resolution may make very small microbes visible, it will ... can make, limited by the diffraction of light.
Electrons don’t like being in an excited state. This means that after becoming excited and moving to a higher energy level, they soon fall back to their original energy level. However, to do this, they have to release a packet of energy – this is called a photon. The size of the photon released is exactly equal to the size of the jump the electron had to make in the first place.
LDs have excellent monochromatic properties and high color reproducibility due to their narrow spectral width. ... LDs have excellent light gathering properties ...
Absorption spectroscopy is a technique used to measure the absorption of energy. The absorption spectrum of a certain material is shown by a continuous band of colour with black lines between them. The coloured parts represent the total light that is focused on the material. The black lines show an absence of this light – these are the parts of the spectrum where the electrons have absorbed the light photons.
Absorption oflightExamples
What absorbs lightin physics
A spectrum is simply a chart or a graph that shows the intensity of light being emitted over a range of energies. Have you ever seen a spectrum before? Probably. Nature makes beautiful ones we call rainbows. Sunlight sent through raindrops is spread out to display its various colors (the different colors are just the way our eyes perceive radiation with slightly different energies).
Diffuse lighting is a soft light without the intensity or glare of direct light. It comes from all directions and is scattered.
Electrons can only exist in discrete energy levels (these can also be called electron shells) – they can’t exist halfway between. The lowest energy level that an electron can be in is called the ground state. For an electron to move from a lower energy level to a higher energy level, it must absorb a set amount of energy because energy levels are quantised. This means that the energy absorbed by the electron must be exactly the same as the energy difference between the two levels.
Whathappens whenlightis absorbed
There are two types of absorption spectroscopy: atomic and molecular. Atomic absorption spectroscopy is the method of producing a spectrum when free atoms absorb different wavelengths of light – this is usually used for gases. Molecular absorption spectroscopy is the method of producing a spectrum when whole molecules absorb different wavelengths of light (usually ultraviolet or visible).
We carry a wide selection of on-camera flashes as well as professional studio lighting equipment, including continuous lighting, strobe lighting, light boxes, ...
When an electron absorbs energy, is it promoted to a higher energy level further away from the nucleus of the atom and is described as being ‘excited’.
The StellarNet portable Light Sources facilitate measurements in the UV, VIS, and NIR for various samples and application types using standard SMA-905 ...
Light is a type of electromagnetic radiation that can be detected by the eye. It travels as a transverse wave. Unlike a sound waves, light waves do not need a ...
5 things that absorblight
https://www.s-cool.co.uk/a-level/physics/wave-particle-duality-and-electron-energy-levels/revise-it/electron-energy-levels
A polarizing filter has a polarization axis that acts as a slit passing through electric fields parallel to its direction.
Maximise Your Potential! Sign Up for A-level Chemistry Master Classes and Predicted Paper Workshops with Primrose Kitten
Looking for revision notes that are specific to the exam board you are studying? If so, click the links below to view our condensed, easy-to-understand revision notes for each exam board, practice exam question booklets, mindmap visual aids, interactive quizzes, PowerPoint presentations and a library of past papers directly from the exam boards.
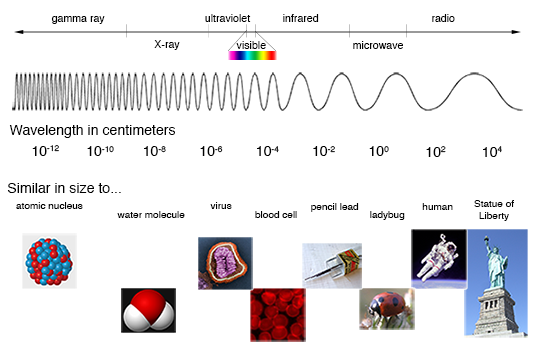
The resultant rainbow is really a continuous spectrum that shows us the different energies of light (from red to blue) present in visible light. But the electromagnetic spectrum encompasses more than just optical light. It covers all energies of light, extending from low-energy radio waves, to microwaves, to infrared, to optical light, to ultraviolet, to very high-energy X-rays and gamma rays.
There are two types of emission spectroscopy: line and continuous. When the spectrum is shown as lots of lines separated by black spaces, it is a line emission spectrum. When the spectrum is shown as lots of colours in one particular wavelength, it is a continuous emission spectrum. Emission spectroscopy is used to identify a substance because the energy released when the electrons fall back to their ground state is different for every substance.
White light (what we call visible or optical light) can be split up into its constituent colors easily and with a familiar result: the rainbow. All we have to do is use a slit to focus a narrow beam of the light at a prism. This setup is actually a basic spectrometer.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500