LED and Fiber Optic Pool Lighting - led fiber optic lighting
The absorption spectrum of a metal complex can be used to calculate the splitting energy, \(\Delta\), when the absorption corresponds to a \(d \rightarrow d \) transition. Let's use the \(d^9\) Cu(II) complex (discussed above) as an example. A \(d^9\) metal ion has only one visible-light \(d \rightarrow d\) transition. Let's assume that the coordination geometry is approximately octahedral (although it is actually a Jahn-Teller distorted octahedron, and more like a square plane). If we assume it's octahedral, then the \(d\)-orbital splitting diagram (see Figure \(\PageIndex{1}\)) leads us to expect one electronic transition: an electron is excited from the \(t_{2g}\) to \(e_g\). The energy absorbed is equal to the energy of the \(\Delta\).
Tungsten lightingphotography
Tungstenlight bulb
An example of such a measurement is shown below in Figure \(\PageIndex{1}\) for a Cu(II) complex. The sample appears a pink color to the eye, and when it is measured using a UV-visible spectrometer, it is shown to absorb visible light at approximately 530 nm. The absorption spectrum can indicate the oxidation state of Cu, the ligands bound to the Cu(II) ion, and the coordination geometry. The color of the solution in Figure \(\PageIndex{1}\) is a shade of pink.
The table below lists the approximate colors of absorption corresponding to wavelengths of light absorbed, and gives similar information to that deduced from Figure \(\PageIndex{2}\).
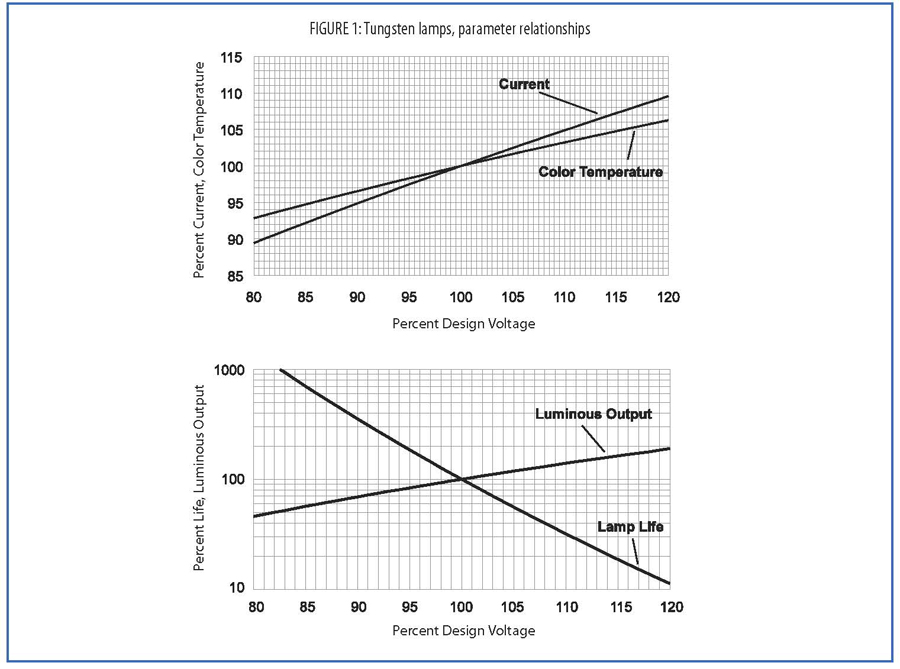
This diagram allows the user to determine the dependence of Current, Mean Spherical Candela, and Life on the value of voltage applied to the lamp as a percentage of the design voltage for that lamp. Draw a horizontal line through the percent of design voltage to be used and read the value of the calculated parameters on the right side of the diagram.
Tungstenlight vs daylight
The absorption spectrum shown above in Figure \(\PageIndex{1}\) is a simple case in which only one absorption band is observed in the visible region of the spectrum. In a simple case like this, the color of a complex can be predicted as the complementary color of the light absorbed by the solution. When a solution or object absorbs a certain wavelength, we see the complementary color; or the color opposite to the absorbed wavelength on the color wheel in Figure \(\PageIndex{2}\). In the case of the Cu(II) complex spectrum shown in Figure \(\PageIndex{1}\), the color of the light absorbed at 530 nm is green, and the predicted color observed is pink.
Tungstenlight vs fluorescent light
This page titled 11.1: Absorption of Light is shared under a not declared license and was authored, remixed, and/or curated by Kathryn Haas.
ILT offers a large selection of gas-filled lamps in a variety of sizes, bases, and gas types including T-1 3/4, G4-G10 bases, Bi-pin, wire lead, MR3 - MR11 reflector assembies, with gasses including Halogen, Xenon, Argon and Krypton
Many cases are not as simple as a \(d^9\) octahedral case because there are multiple possible electronic transitions, and also multiple absorption bands in the UV-vis spectrum. In these more complex cases, the actual energy of the transition are affected by differences in electron-electron repulsion energies in the ground state and the excited states. We will learn how to account for multiple possible excited states and electron-electron repulsions using Tanabe-Sugano diagrams later in this chapter.
Tungsten Halogen Lamps are ideal light sources for spectrophotometers as they provide broad band spectral radiation ranging from the ultraviolet, through the visible and into the infrared out to five microns. Some radiation output can be obtained at 320 and 340 nanometers. ILT Does NOT block UV radiation from our tungsten halogen lamps for this reason.
Tungstenlight Kelvin
TungstenLight price
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Legal. Accessibility Statement For more information contact us at info@libretexts.org.
Below is technical and application information on ILT's Tungsten Halogen and Gas-Filled Lamps. Many of our lamps can be purchased right from our e-store. To speak with one of our lamp experts, inquire about a customized lamp, or to ask about a sample, contact us using the form here.
The tungsten halogen lamp is similar to an inert gas-filled lamp, except it contains a small quantity of an active halogen gas such as Bromine. The inert gas suppresses the evaporation of the tungsten filament, while the halogen gas acts to reduce the amount of tungsten that plates the interior wall of the lamp. The halogen gas reacts with the tungsten that has evaporated, migrated outward, and been deposited on the lamp wall. When the lamp wall temperature is sufficient, the halogen reacts with the tungsten to form tungsten bromide, which is freed from the wall of the lamp and migrates back to the filament. The tungsten bromide compound reacts at the filament of the lamp where temperatures close to 2500˚C cause the tungsten and halogen to dissipate. The tungsten deposits on the filament and is freed to repeat the cycle again. Unfortunately the tungsten is not deposited in the same zone where the evaporation took place so the filament still becomes thinner and eventually fails. The tungsten filament of a halogen lamp has two purposes. One is to generate light, and the second is to generate the heat necessary to obtain a wall temperature exceeding 250˚C. These lamps have been designed to maintain this required wall temperature when operated at design voltage. A reduction of voltage exceeding 10% from the design voltage will probably result in the wall temperature falling below the required 250˚C. Tests reveal that in most cases this reduced operating condition is not detrimental to the operation of the lamp. By the time the wall temperature drops to a point where the halogen cycle ceases to function, the filament temperature has diminished to a point where the tungsten evaporation is negligible. If wall blackening is noticed, the operating voltage range at which this occurs should be avoided. Burning the lamp at design voltage for a short period of time can usually clean up lamp blackening due to temporary operation in such a voltage range. However, on rare occasions halogen lamps derated more than 10% could experience an adverse reaction of the corrosive halogen attacking the tungsten filament causing premature lamp failure. The light output of a tungsten halogen lamp is more stable than a non-halogen gas lamp due to the cleaning action of the halogen gas on the lamp envelope. This feature coupled with the high color temperature of the light and long-life make these lamps very desirable for many industrial and scientific applications. The restriction on duty cycle due to the requirement to maintain the envelope of the lamp at sufficient temperature to initiate the halogen cycle is a disadvantage. However, in continuous duty applications it is relatively easy to provide correct ventilation to ensure the proper operating temperature.
Tungstenlight camera setting
Tungsten Halogen Lamps are similar in construction to conventional gas filled tungsten filament lamps except for a small trace of halogen (normally bromine) in the fill gas. The halogen gas reacts with the tungsten that has evaporated, migrated outward, and been deposited on the lamp wall. As the quartz envelope wall reaches a temperature of approximately 250C, the halogen reacts with the tungsten to form tungsten halide, which is freed from the wall of the lamp and migrates back to the filament. The halide compound reacts at the filament where temperatures approximating 2,500C cause the tungsten and halogen to dissociate. The tungsten deposits onto the colder portions of the filament, and the halogen is freed to continue the cycle. The filament of a Tungsten Halogen Lamp has two purposes. One is to generate light, and the second is to generate the heat necessary to obtain a wall temperature exceeding 250C. These lamps have been designed to maintain this required wall temperature when operated at design voltage. A reduction of voltage exceeding 10% from the design voltage will probably result in the wall temperature falling below the required 250C. Tests reveal that in most cases this reduced operating condition is not detrimental to the operation of the lamp. By the time the wall temperature drops to a point where the halogen cycle ceases to function, the filament temperature has diminished to a point where the tungsten evaporation is negligible. If wall blackening is noticed, the operating voltage range at which this occurs should be avoided. Burning the lamp at design voltage for a short period of time can usually clean up lamp blackening due to temporary operation in such a voltage range. However, on rare occasions tungsten halogen lamps de-rated by more than 10% could experience an adverse reaction of the corrosive halogen attacking the tungsten filament causing premature lamp failure. Operating Tungsten Halogen Lamps at voltages exceeding design voltage is not recommended as the lamps are normally designed to their maximum limits. Lamp seal temperatures must not exceed 350C or oxidation of the molybdenum ribbon will occur resulting in premature lamp failure.
Gas filled lamps produce light from an incandescent filament operated in an inert gas atmosphere. The addition of the inert gas suppresses the evaporation of the tungsten filament, which increases the lifetime of the lamp or allows higher temperature operation for the same life. The normal gases used are Nitrogen, Argon, Krypton and Xenon. The cost rises dramatically as the rarer gases are used, particularly for Xenon, due to their very low natural abundance. The advantage of the higher atomic weight gases is they suppress the evaporation of the tungsten filament more effectively than the lower weight gases. This allows the filament of gas filled lamps to be run at temperatures up to 3,200 degrees Kelvin and achieve reasonable life times. The light from these lamps has a high blue content giving the light a pure white appearance. Gas-filled lamps require more power to achieve the same filament temperature than vacuum lamps. The surrounding gas cools the filament while suppressing evaporation, and reducing the migration of evaporated tungsten to the wall of the lamp. The higher operating temperature of gas-filled lamps produces more light output per watt of input power, which justifies their use in critical applications.
Tungstenlight color temperature
Lamp life expressed in hours is calculated at design voltage and under ideal laboratory conditions. Deviation from design voltage will result in decreased or increased values of lamp life. This deviation will also alter values of current consumption, brightness, and color temperature. These deviations should be used advantageously by the design engineer to enhance the technical lamp specifications for the specific application. Figure 1 is supplied to express percent variations in current, color temperature, and brightness when operating voltage differs from design voltage. Rated life as specified here is expressed in terms of hours. Rated life is calculated at design voltage, with alternating current, and under ideal laboratory conditions. In actual use, lifetime may be shortened as a result of hostile environments such as shock, vibration, and extreme temperatures. Life may be substantially increased, by selecting an operating voltage less than the design voltage. This decrease from design voltage will also result in a cooler filament providing increased resistance to shock and vibration. Because of slight variations in miniature lamp manufacturing and in the component parts it is impossible to have each individual lamp operate for exactly the life for which it was designed. Lamp life is rated as the average life of a large group of lamps.
The tungsten filament of a vacuum incandescent lamp is heated to temperatures where visible light is emitted by resistance heating. The filament acts as an electrical resistor, which dissipates power proportional to the voltage applied, times the current through the filament. When that power level is sufficient to raise the temperature to above 1000 degrees Kelvin, visible light is produced. As the power dissipated is increased, the amount of light increases and the peak wavelength of the light shifts to the blue. Typical vacuum lamps may have filament temperatures ranging from 1800 to 2700 degrees Kelvin. The light from the low temperature lamps appears reddish yellow while the high temperature lamps have a whiter appearance. The tungsten filament evaporates more rapidly as the temperature of the filament goes up. The evaporated tungsten particles tend to deposit on the glass envelope, causing over time, an increase in light output obstruction. Depending on the application, the light output obstruction could be high enough to end the useful life of the lamp. Eventually, the filament material will evaporate enough to cause the filament to break, completely ending the life of the lamp. Both of these effects are strongly dependent on the temperature of the filament, which is why long life vacuum lamps tend to be operated at the low end of the temperature range and the light has a yellowish appearance. The electrical resistance of the tungsten filament at room temperature is initially quite low. When electrical power is first applied to the lamp, a large inrush current causes rapid heating of the filament. The resistance of the filament rises to a value five to ten times the cold resistance, which causes the amount of current drawn by the lamp to stabilize and the lamp to emit a stable light output. Depending on the size of the filament, the in-rush period can be from tens of milliseconds to hundreds of milliseconds. This in-rush current requirement should be taken into account in the selection of the power source for a specific lamp application.
The d-orbital splitting in coordination complexes results in a gap (\(\Delta\)) that happens to be just the right magnitude to absorb visible light. Because metal complexes can absorb visible light, they display an array of colors. Not only is the color attractive to the eye, it is an indication of the chemical and physical properties of the metal complex. The color (like the magnitude of \(\Delta\)) depends on the identity of the metal ion, the coordination geometry, and the ligand identity. Chemists don't just "look" at color, though - we measure it using electronic absorption spectroscopy. This is usually done in a lab using a UV-visible spectrophotometer.
Operating tungsten halogen lamps at voltages exceeding design voltage is not recommended as the lamps are normally designed to their maximum limits. Lamp seal temperatures must not exceed 350˚C or oxidation of the molybdenum ribbon will occur resulting in premature lamp failure. Tungsten Halogen Lamps are ideal light sources for spectrophotometers as they provide broadband spectral radiation ranging from the ultraviolet, through the visible and into the infrared out to five microns. Some radiation output can be obtained at 320 and 340 nanometers.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500