What is Bias Lighting and Why Do I Need It? - backlighting lights
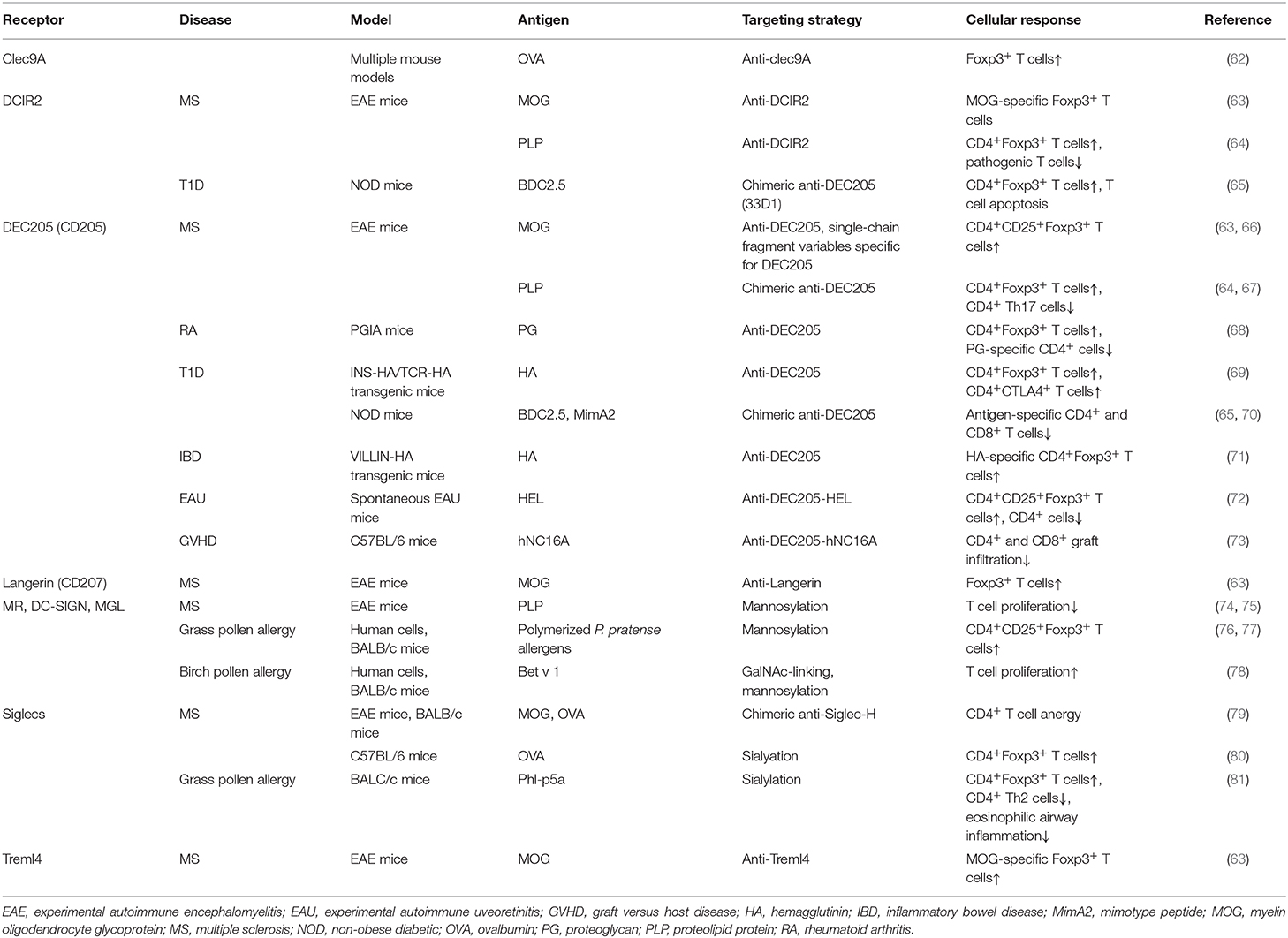
Figure 2. In vivo targeting of receptors on DCs against immunopathologies. Different receptors on dendritic cells (DCs) could be targeted using either antigen-antibody fusion compounds or carbohydrate-modified antigens. Brown arrows: the cDC1 and cDC2 subsets express a variety of receptors in both humans (purple boxes) and mice (blue boxes), which recognize and internalize fusion antibodies coupled with antigens, specific for a DC receptor and mediate the deletion of autoreactive T cells, induction of T cell anergy, generation of antigen-specific Foxp3+ Tregs and expansion of natural Tregs (These tolerogenic responses are abrogated if fusion antibodies are administered together with adjuvants like PolyI:C). The generation of either antigen-specific Foxp3+ Tregs or promotion of natural Tregs expansion depends of the receptor being targeted. Green arrows: Fusion antibodies against Siglecs, coupled with antigens or sialic acid-modified antigens bind to Siglec receptors on DCs and induce anti-inflammatory signals that result in the induction of Foxp3+ T cells, T cell anergy and the inhibition of Th1 responses. Red arrows: Targeting the MR, DC-SIGN and MGL on DCs with antigens conjugated to specific glycan moieties, result in the polarization of Th2 responses in allergy to Th1 responses and the induction of Foxp3+ T cells. Moreover, this interaction promotes the production of IgG4 blocking antibodies and mediates the reduction of IgE secretion. CLEC9A, C-type lectin domain family 9 member A; Treml4, The Triggering Receptor Expressed on Myeloid cells-like 4; DCIR, dendritic cell immunoreceptor; CLEC12A, C-type lectin domain family 12 member A; cDC1, Conventional type 1 dendritic cells; cDC2, Conventional type 2 dendritic cells; Siglec, Sialic acid-binding immunoglobulin-type lectins; MR, Mannose receptor; DC-SIGN, Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin; MGL, macrophage galactose-type C-type lectin. Langerin*: Induced expression.
17. Brown CC, Gudjonson H, Pritykin Y, Deep D, Lavallée VP, Mendoza A, et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell. (2019) 179:846–63.e24. doi: 10.1016/j.cell.2019.09.035
136. Spence S, Greene MK, Fay F, Hams E, Saunders SP, Hamid U, et al. Targeting siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci Transl Med. (2015) 7:303ra140. doi: 10.1126/scitranslmed.aab3459
105. Yang F, Fan X, Huang H, Dang Q, Lei H, Li Y. A single microorganism epitope attenuates the development of murine autoimmune arthritis: regulation of dendritic cells via the mannose receptor. Front Immunol. (2018) 9:1528. doi: 10.3389/fimmu.2018.01528
The early pioneers of microscopy opened a window into the invisible world of microorganisms. But microscopy continued to advance in the centuries that followed. In 1830, Joseph Jackson Lister created an essentially modern light microscope. The 20th century saw the development of microscopes that leveraged nonvisible light, such as fluorescence microscopy, which uses an ultraviolet light source, and electron microscopy, which uses short-wavelength electron beams. These advances led to major improvements in magnification, resolution, and contrast. By comparison, the relatively rudimentary microscopes of van Leeuwenhoek and his contemporaries were far less powerful than even the most basic microscopes in use today. In this section, we will survey the broad range of modern microscopic technology and common applications for each type of microscope.
Similar to an STM, AFMs have a thin probe that is passed just above the specimen. However, rather than measuring variations in the current at a constant height above the specimen, an AFM establishes a constant current and measures variations in the height of the probe tip as it passes over the specimen. As the probe tip is passed over the specimen, forces between the atoms (van der Waals forces, capillary forces, chemical bonding, electrostatic forces, and others) cause it to move up and down. Deflection of the probe tip is determined and measured using Hooke’s law of elasticity, and this information is used to construct images of the surface of the specimen with resolution at the atomic level (Figure \(\PageIndex{16}\)).
The item being viewed is called a specimen. The specimen is placed on a glass slide, which is then clipped into place on the stage(a platform) of the microscope. Once the slide is secured, the specimen on the slide is positioned over the light using the x-y mechanical stage knobs. These knobs move the slide on the surface of the stage, but do not raise or lower the stage. Once the specimen is centered over the light, the stage position can be raised or lowered to focus the image. The coarse focusing knob is used for large-scale movements with 4⨯ and 10⨯ objective lenses; the fine focusing knob is used for small-scale movements, especially with 40⨯ or 100⨯ objective lenses.
An opaque light stop inserted into a brightfield microscope is used to produce a darkfield image. The light stop blocks light traveling directly from the illuminator to the objective lens, allowing only light reflected or refracted off the specimen to reach the eye.
Model, Dimension, Description, PDF, Price. RLF3-45-050-1-X, Diffused Ring Light illumination OD: 56.4mm, ID: 10mm, Angle 45° Color (R.G.B.W), Power 2.90W, ...
73. Ettinger M, Gratz IK, Gruber C, Hauser-Kronberger C, Johnson TS, Mahnke K, et al. Targeting of the hNC16A collagen domain to dendritic cells induces tolerance to human type XVII collagen. Exp Dermatol. (2012) 21:395–8. doi: 10.1111/j.1600-0625.2012.01474.x
Dec 6, 2024 — Does anyone have experience/tips with using ARTIQ to control Allied Vision Alvium Cameras? Reply. Write a Reply... Loading.
114. van Vliet SJ, van Liempt E, Saeland E, Aarnoudse CA, Appelmelk B, Irimura T, et al. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol. (2005) 17:661–9. doi: 10.1093/intimm/dxh246
12. Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther Adv Vaccines. (2014) 2:159–82. doi: 10.1177/2051013614541440
75. Luca ME, Kel JM, Van Rijs W, Drijfhout JW, Koning F, Nagelkerken L. Mannosylated PLP139-151 induces peptide-specific tolerance to experimental autoimmune encephalomyelitis. J Neuroimmunol. (2005) 160:178–87. doi: 10.1016/j.jneuroim.2004.11.014
56. McGreal EP, Miller JL, Gordon S. Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol. (2005) 17:18–24. doi: 10.1016/j.coi.2004.12.001
A darkfield microscope is a brightfield microscope that has a small but significant modification to the condenser. A small, opaque disk (about 1 cm in diameter) is placed between the illuminator and the condenser lens. This opaque light stop, as the disk is called, blocks most of the light from the illuminator as it passes through the condenser on its way to the objective lens, producing a hollow cone of light that is focused on the specimen. The only light that reaches the objective is light that has been refracted or reflected by structures in the specimen. The resulting image typically shows bright objects on a dark background (Figure \(\PageIndex{3}\))
61. Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, et al. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. (2000) 151:673–83. doi: 10.1083/jcb.151.3.673
97. Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SCM, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-κB activation through Raf-1 and Syk. Nat Immunol. (2009) 10:203–13. doi: 10.1038/ni.1692
112. Manzano AI, Javier Cañada F, Cases B, Sirvent S, Soria I, Palomares O, et al. Structural studies of novel glycoconjugates from polymerized allergens (allergoids) and mannans as allergy vaccines. Glycoconj J. (2016) 33:93–101. doi: 10.1007/s10719-015-9640-4
63. Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest. (2013) 123:844–54. doi: 10.1172/JCI65260
Feb 2, 2023 — With this minimal reproduction, raising Indirect Light clamping from 3 to 10 (Blender default) seems to get rid of the issue completely.
Besides the immature and semi-mature tolDCs, several tissue-specific DCs exhibit inherent tolerogenic properties, including those in the skin and intestines. In the skin, Langerhans cells (LCs), which are characterized by the expression of Langerin (CD207), CD1a, E-Cadherin, CD39, FcεRI, and Birbeck granules, are the sole tissue-resident DC population in the epidermis (29). LCs constantly migrate from the skin to draining lymph nodes, even in steady-state conditions, and have been implicated in both immunogenic as well as tolerogenic immune reactions (30–32). In contrast, CD14+(CD141+) dermal DCs constitutively secrete the anti-inflammatory cytokine IL-10 and are prone to induce T cell anergy and Tregs that inhibit skin inflammation (33, 34). The ability to produce extensive levels of IL-10 is shared with CD14+CD16+CD141+CD163+ DCs isolated from peripheral blood, identified by Gregori and colleagues, which might correspond to the DC3 subset expressing the same surface markers (16, 35). The same group has shown that these cells express the surface receptors HLA-G, ILT2, ILT3, and ILT4 and have the potency to induce type 1 Tregs (Tr1) in vitro (36–38). In the intestines, the main subset involved in oral tolerance during steady state conditions are the CD103+ DCs in the lamina propia and mesenteric lymph nodes. They are able to prime Tregs in gut lymphoid tissues through the production of TGF-β and RA (39–42). Additionally, CD103+ DCs express high levels of RALDH2, converting vitamin A to RA which enhances Treg induction (29). These studies demonstrate that various tissues contain specific subsets of tolDCs, emphasizing the power of the immune system to adapt to specific environmental factors functioning to maintain immune homeostasis during tissue-specific circumstances.
It means the internal power cell is charging. The blue light fills the circle clockwise as the Wired Doorbell Plus charges its internal power cell.
DC-SIGN and MR are other members of the CLR family which recognize several mannose and fucose-containing structures, present on many antigens (49) and activate signaling pathways in CLR-expressing cells (44, 52, 60). These receptors are widely expressed on DCs and have been extensively exploited in several fields as potential targets for immunotherapy (89–91). Previously, antibody-mediated CLR targeting has been the most studied strategy for antigen delivery and activation of DCs in vivo, but in recent years glycan-based targeting approaches are gaining increasing attention (Table 1) (89, 90). Compared to antibody-mediated targeting, in glycan-based targeting, the spatial orientation of displayed carbohydrate CLR ligands can be varied more easily according to the distances between receptor binding sites thereby enhancing receptor-ligand binding and subsequent signaling (89). DC-SIGN is exclusively expressed on immature DCs and shows properties that are often, but not always, associated with Th2 polarization, suppression of inflammation and/or induction of regulatory immune response inhibiting pro-inflammatory Th1/Th17 immunity, especially when it recognizes helminth or allergen associated antigens (92–96). Interestingly, binding of the mycobacterial cell wall component Mannose-capped Lipoarabinomannan (ManLAM) to DC-SIGN inhibits DC maturation and induces IL-10 production (97). Also, the use of fucosylated ligands targeting DC-SIGN biases immune responses toward anti-Th1 responses, with an enhanced Th2 response, and has been shown to ameliorate different autoimmune conditions pre-clinically (92, 98). For instance, exposure of NOD mice to fucose-containing schistosome antigens inhibited the development of type 1 diabetes. This finding is in agreement with reports that have shown that such glycan-CLR signaling can induce a regulatory T cell phenotype having IL-10 and TGF-β production (99), which could explain the observed prevention of the development of autoimmunity in these mice (98, 100).
84. Maksimow M, Miiluniemi M, Marttila-Ichihara F, Jalkanen S, Hänninen A. Antigen targeting to endosomal pathway in dendritic cell vaccination activates regulatory T cells and attenuates tumor immunity. Blood. (2006) 108:1298–305. doi: 10.1182/blood-2005-11-008615
85. Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. (2002) 196:1627–38. doi: 10.1084/jem.20021598
Fluorescence microscopes are especially useful in clinical microbiology. They can be used to identify pathogens, to find particular species within an environment, or to find the locations of particular molecules and structures within a cell. Approaches have also been developed to distinguish living from dead cells using fluorescence microscopy based upon whether they take up particular fluorochromes. Sometimes, multiple fluorochromes are used on the same specimen to show different structures or features.
Signals such as pathogen-associated molecular patterns (PAMPS) from pathogens, damage associated molecular patterns (DAMPS) from inflammation, and self-associated molecular patterns (SAMPS) can be recognized by pattern recognition receptors (PRRs) on the surface of DCs (21, 43). C-type lectins (CLRs) and Sialic-acid binding immunoglobulin-type lectins (Siglecs) are families of PRRs equipped with a carbohydrate recognition domain that specifically recognizes glycan moieties on host cells, pathogens, as well as innocuous antigens such as allergens (21, 43–46).
Currently, use of two-photon microscopes is limited to advanced clinical and research laboratories because of the high costs of the instruments. A single two-photon microscope typically costs between $300,000 and $500,000, and the lasers used to excite the dyes used on specimens are also very expensive. However, as technology improves, two-photon microscopes may become more readily available in clinical settings.
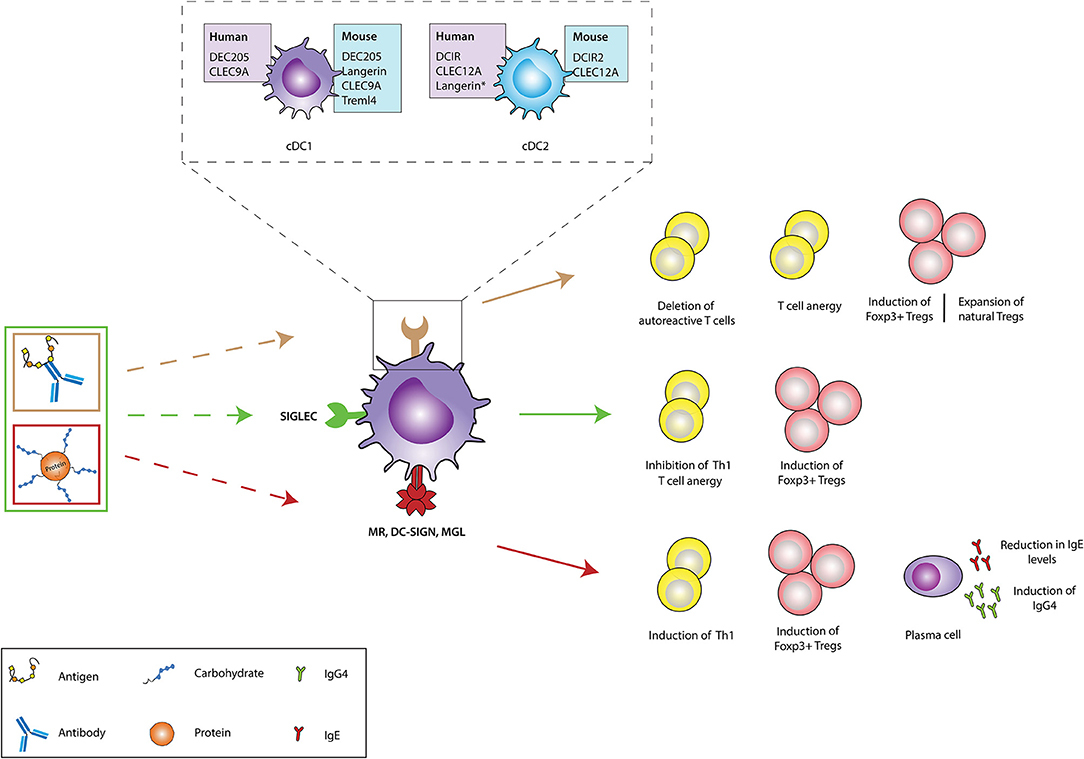
Immunomodulatory agents such as vitamin D3, retinoic acid, rapamycin, dexamethasone, corticosteroids, ligands of the aryl hydrocarbon receptor (AhR), or specific cytokines (IL-10, TGFβ) have been key in determining the existence and function of tolerogenic DCs (tolDCs) ex vivo (Figure 1) (5–7). These tolDCs can induce tolerance through various mechanisms, including the induction of Tregs, autoreactive T cell anergy and apoptosis, and could be used in tolerizing immunotherapies (6, 8, 9). Ex vivo tolDC immunotherapies are based on re-education of patient-derived DCs to a tolerizing phenotype and the subsequent reinfusion into the body, where they suppress inflammatory immune responses (Figure 1). The first clinical study utilizing tolerogenic DCs (tolDCs) for the treatment of autoimmune diseases was performed in 2011 in adult type I diabetes (T1D) patients. Since then, phase I and II clinical trials have been conducted for T1D, rheumatoid arthritis (RA), Crohn's disease, and multiple sclerosis (MS) (5), but also for kidney and liver transplant recipients (8–10). However, due to the personalized, laborious, and expensive nature of ex vivo-generated tolDCs, new approaches for inducing tolDCs in vivo are being developed.
93. Favoretto BC, Casabuono AAC, Portes-Junior JA, Jacysyn JF, Couto AS, Faquim-Mauro EL. High molecular weight components containing N-linked oligosaccharides of Ascaris suum extract inhibit the dendritic cells activation through DC-SIGN and MR. Mol Immunol. (2017) 87:33–46. doi: 10.1016/j.molimm.2017.03.015
113. Benito-Villalvilla C, Soria I, Pérez-Diego M, Fernández-Caldas E, Subiza JL, Palomares O. Alum impairs tolerogenic properties induced by allergoid-mannan conjugates inhibiting mTOR and metabolic reprogramming in human DCs. Allergy Eur J Allergy Clin Immunol. (2020) 75:648–59. doi: 10.1111/all.14036
Optical microscope
119. Schreibelt G, Klinkenberg LJJ, Cruz LJ, Tacken PJ, Tel J, Kreutz M, et al. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood. (2012) 119:2284–92. doi: 10.1182/blood-2011-08-373944
5. Cauwels A, Tavernier J. Tolerizing strategies for the treatment of autoimmune diseases: from ex vivo to in vivo strategies. Front Immunol. (2020) 11: e674. doi: 10.3389/fimmu.2020.00674
Copyright © 2021 Castenmiller, Keumatio-Doungtsop, van Ree, de Jong and van Kooyk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Lightfield microscopy
A brightfield microscope creates an image by directing light from the illuminator at the specimen; this light is differentially transmitted, absorbed, reflected, or refracted by different structures. Different colors can behave differently as they interact withchromophores (pigments that absorb and reflect particular wavelengths of light) in parts of the specimen. Often, chromophores are artificially added to the specimen using stains, which serve to increase contrast and resolution. In general, structures in the specimen will appear darker, to various extents, than the bright background, creating maximally sharp images at magnifications up to about 1000⨯. Further magnification would create a larger image, but without increased resolution. This allows us to see objects as small as bacteria, which are visible at about 400⨯ or so, but not smaller objects such as viruses.
39. Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β -and retinoic acid-dependent mechanism. J Exp Med. (2007) 204:1757–64. doi: 10.1084/jem.20070590
31. Azukizawa H, Döhler A, Kanazawa N, Nayak A, Lipp M, Malissen B, et al. Steady state migratory RelB+ langerin+ dermal dendritic cells mediate peripheral induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells. Eur J Immunol. (2011) 41:1420–34. doi: 10.1002/eji.201040930
19. Rodrigues PF, Tussiwand R. Novel concepts in plasmacytoid dendritic cell (pDC) development and differentiation. Mol Immunol. (2020) 126:25–30. doi: 10.1016/j.molimm.2020.07.006
127. García-Vallejo JJ, Bloem K, Knippels LMJ, Garssen J, van Vliet SJ, van Kooyk Y. The consequences of multiple simultaneous C-type lectin-ligand interactions: DCIR alters the endo-lysosomal routing of DC-SIGN. Front Immunol. (2015) 6:87. doi: 10.3389/fimmu.2015.00087
The brightfield microscope, perhaps the most commonly used type of microscope, is a compound microscope with two or more lenses that produce a dark image on a bright background. Some brightfield microscopes are monocular (having a single eyepiece), though most newer brightfield microscopes are binocular (having two eyepieces), like the one shown in Figure \(\PageIndex{1}\); in either case, each eyepiece contains a lens called an ocular lens. The ocular lenses typically magnify images 10 times (10⨯). At the other end of the body tube are a set of objective lenses on a rotating nosepiece. The magnification of these objective lenses typically ranges from 4⨯ to 100⨯, with the magnification for each lens designated on the metal casing of the lens. The ocular and objective lenses work together to create a magnified image. The total magnification is the product of the ocular magnification times the objective magnification:
7. Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. (2010) 107:20768–73. doi: 10.1073/pnas.1009201107
10. Aragão-França LS, Rocha VCJ, Cronemberger-Andrade A, Costa FHB, Vasconcelos JF, Athanazio DA, et al. Tolerogenic dendritic cells reduce airway inflammation in a model of dust mite triggered allergic inflammation. Allergy Asthma Immunol Res. (2018) 10:406–19. doi: 10.4168/aair.2018.10.4.406
The feasibility and potential of in vivo strategies lie in the ability of DCs to recognize and internalize antigens through surface receptors that not only route antigens to the antigen processing machinery of DCs for subsequent presentation to T cells but also transmit signals that direct anti-inflammatory immune responses. This allows direct modulation of specific DC subsets due to differential surface receptor expression profiles between them. In vivo DC-targeting has several advantages compared to ex vivo DC-targeting, including fewer hospital visits for the patient, less laborious production methods, and the possibility of large scale production, which is more cost-effective. Additionally, the induction of antigen-specific T cell responses with in vivo DC-targeting strategies reduces the risk of generalized immunosuppression, which is induced during the current ex vivo strategies using only immunosuppressive agents. The main strategies for in vivo tolDC generation take advantage of modalities binding to specific endocytic receptors on DC surfaces, ensuring the delivery of antigen of interest into the antigen-processing machinery (Figure 1) (11). Antigens could either be directly coupled to antibodies (11) or loaded on nanoparticles or in liposomes, reviewed elsewhere (12). Another strategy being explored in this regard involves chemically conjugating antigens with specific glycan structures which are ligands for DC surface receptors. In this review we discuss the different DC-subsets used for targeting, the receptors expressed on their surface that have potential to induce tolerogenic signals (but might not be inherently tolerogenic), and the current state of research in their use for the treatment of auto-immune or allergic diseases.
98. Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. (2009) 39:1098–107. doi: 10.1002/eji.200838871
Darkfield microscopy
Wound infections like Cindy’s can be caused by many different types of bacteria, some of which can spread rapidly with serious complications. Identifying the specific cause is very important to select a medication that can kill or stop the growth of the bacteria.
Keywords: dendritic cell, tolerance, immunotherapy, surface receptors, C-type lectins, Siglecs, allergy, auto immune diseases
116. Bigley V, McGovern N, Milne P, Dickinson R, Pagan S, Cookson S, et al. Langerin-expressing dendritic cells in human tissues are related to CD1c + dendritic cells and distinct from Langerhans cells and CD141 high XCR1 + dendritic cells. J Leukoc Biol. (2015) 97:627–34. doi: 10.1189/jlb.1hi0714-351r
101. Taylor PR, Gordon S, Martinez-Pomares L. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol. (2005) 26:104–10. doi: 10.1016/j.it.2004.12.001
59. Lübbers J, Rodríguez E, van Kooyk Y. Modulation of immune tolerance via siglec-sialic acid interactions. Front Immunol. (2018) 9:2807. doi: 10.3389/fimmu.2018.02807
134. Álvarez B, Nieto-Pelegrín E, Martínez de la Riva P, Toki D, Poderoso T, Revilla C, et al. Characterization of the porcine CLEC12A and analysis of its expression on blood dendritic cell subsets. Front Immunol. (2020) 11:863. doi: 10.3389/fimmu.2020.00863
Nov 2, 2016 — I just saw this new product called CMRA, a band for your Apple Watch that has not one but two cameras built into the strap.
46. Duan S, Paulson JC. Siglecs as immune cell checkpoints in disease. Annu Rev Immunol. (2020) 38:365–95. doi: 10.1146/annurev-immunol-102419-035900
Widefield microscope
This figure compares a brightfield image (left) with a phase-contrast image (right) of the same unstained simple squamous epithelial cells. The cells are in the center and bottom right of each photograph (the irregular item above the cells is acellular debris). Notice that the unstained cells in the brightfield image are almost invisible against the background, whereas the cells in the phase-contrast image appear to glow against the background, revealing far more detail. (credit: “Clearly kefir”/Wikimedia Commons)
Bar Lighting | Machine vision lenses such as fixed focal, telecentric, macro, line scan, and zoom lenses are designed and developed for various ...
120. Crozat K, Tamoutounour S, Vu Manh T-P, Fossum E, Luche H, Ardouin L, et al. Cutting edge: expression of XCR1 defines mouse lymphoid-tissue resident and migratory dendritic cells of the CD8α + type. J Immunol. (2011) 187:4411–5. doi: 10.4049/jimmunol.1101717
100. Ruyssers NE, De Winter BY, De Man JG, Loukas A, Herman AG, Pelckmans PA, et al. Worms and the treatment of inflammatory bowel disease: are molecules the answer? Clin Dev Immunol. (2008) 2008:567314. doi: 10.1155/2008/567314
A DIC image of Fonsecaea pedrosoi grown on modified Leonian’s agar. This fungus causes chromoblastomycosis, a chronic skin infection common in tropical and subtropical climates.
60. Figdor CG, Van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and langerhans cells. Nat Rev Immunol. (2002) 2:77–84. doi: 10.1038/nri723
14. Lamendour L, Deluce-Kakwata-nkor N, Mouline C, Gouilleux-Gruart V, Velge-Roussel F. Tethering innate surface receptors on dendritic cells: a new avenue for immune tolerance induction? Int J Mol Sci. (2020) 21:1–15. doi: 10.3390/ijms21155259
One of the most important applications of fluorescence microscopy is a technique called immunofluorescence, which is used to identify certain disease-causing microbes by observing whether antibodies bind to them. (Antibodies are protein molecules produced by the immune system that attach to specific pathogens to kill or inhibit them.) There are two approaches to this technique: direct immunofluorescence assay (DFA) and indirect immunofluorescence assay (IFA). In DFA, specific antibodies (e.g., those that the target the rabies virus) are stained with a fluorochrome. If the specimen contains the targeted pathogen, one can observe the antibodies binding to the pathogen under the fluorescent microscope. This is called a primary antibody stain because the stained antibodies attach directly to the pathogen.
89. Lepenies B, Lee J, Sonkaria S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv Drug Deliv Rev. (2013) 65:1271–81. doi: 10.1016/j.addr.2013.05.007
The microscope transmits an excitation light, generally a form of EMR with a short wavelength, such as ultraviolet or blue light, toward the specimen; the chromophores absorb the excitation light and emit visible light with longer wavelengths. The excitation light is then filtered out (in part because ultraviolet light is harmful to the eyes) so that only visible light passes through the ocular lens. This produces an image of the specimen in bright colors against a dark background.
104. Keler T, Ramakrishna V, Fanger MW. Mannose receptor-targeted vaccines. Expert Opin Biol Ther. (2004) 4:1953–62. doi: 10.1517/14712598.4.12.1953
65. Price JD, Hotta-Iwamura C, Zhao Y, Beauchamp NM, Tarbell KV. DCIR2+ cDC2 DCs and Zbtb32 restore CD4+ T-cell tolerance and inhibit diabetes. Diabetes. (2015) 64:3521–31. doi: 10.2337/db14-1880
SEMs form images of surfaces of specimens, usually from electrons that are knocked off of specimens by a beam of electrons. This can create highly detailed images with a three-dimensional appearance that are displayed on a monitor (Figure \(\PageIndex{12}\)). Typically, specimens are dried and prepared with fixatives that reduce artifacts, such as shriveling, that can be produced by drying, before being sputter-coated with a thin layer of metal such as gold. Whereas transmission electron microscopy requires very thin sections and allows one to see internal structures such as organelles and the interior of membranes, scanning electron microscopy can be used to view the surfaces of larger objects (such as a pollen grain) as well as the surfaces of very small samples (Figure \(\PageIndex{13}\)). Some EMs can magnify an image up to 2,000,000⨯.1
35. Comi M, Avancini D, Santoni de Sio F, Villa M, Uyeda MJ, Floris M, et al. Coexpression of CD163 and CD141 identifies human circulating IL-10-producing dendritic cells (DC-10). Cell Mol Immunol. (2020) 17:95–107. doi: 10.1038/s41423-019-0218-0
77. Soria I, Alvarez J, Manzano AI, López-Relaño J, Cases B, Mas-Fontao A, et al. Mite allergoids coupled to nonoxidized mannan from Saccharomyces cerevisae efficiently target canine dendritic cells for novel allergy immunotherapy in veterinary medicine. Vet Immunol Immunopathol. (2017) 190:65–72. doi: 10.1016/j.vetimm.2017.07.004
126. Erdmann H, Steeg C, Koch-Nolte F, Fleischer B, Jacobs T. Sialylated ligands on pathogenic Trypanosoma cruzi interact with Siglec-E (sialic acid-binding Ig-like lectin-E). Cell Microbiol. (2009) 11:1600–11. doi: 10.1111/j.1462-5822.2009.01350.x
64. Tabansky I, Keskin DB, Watts D, Petzold C, Funaro M, Sands W, et al. Targeting DEC-205-DCIR2+ dendritic cells promotes immunological tolerance in proteolipid protein-induced experimental autoimmune encephalomyelitis. Mol Med. (2018) 24:17. doi: 10.1186/s10020-018-0017-6
This diagram of a phase-contrast microscope illustrates phase differences between light passing through the object and background. These differences are produced by passing the rays through different parts of a phase plate. The light rays are superimposed in the image plane, producing contrast due to their interference.
Citation: Castenmiller C, Keumatio-Doungtsop B-C, van Ree R, de Jong EC and van Kooyk Y (2021) Tolerogenic Immunotherapy: Targeting DC Surface Receptors to Induce Antigen-Specific Tolerance. Front. Immunol. 12:643240. doi: 10.3389/fimmu.2021.643240
Although the immune system encounters many innocuous antigens, including self-antigens and allergens, the chance to develop autoimmune or allergic diseases is relatively small due to the phenomenon of “natural tolerance.” Natural tolerance is achieved through the presence of tolerance-inducing DCs located both centrally and in the periphery. Central tolerance induction is mediated by thymic epithelial cells and thymic DCs, which regulate negative selection of autoreactive T cells and induction of natural Tregs (23, 24). Even though the specific role of each thymic DC subset in peripheral immune homeostasis remains elusive, thymic pDCs and the Sirpα+ cDC subset have been proposed to contribute to the prevention of allergic or commensal-specific autoimmune diseases, as they originate from the periphery where they encounter many innocuous antigens, followed by migration to the thymus (23–25). On the other hand, peripheral tolerance is mediated by peripheral DCs, preferentially located at the border between the body and the external environment, such as lung, intestine and skin. Steady state or immature DCs were the original identified peripheral tolDCs (26–28). They exhibit low expression of co-stimulatory (CD40, CD80/86) and MHC molecules due to lack of appropriate activation signals and are able to maintain tolerance via deletion of self-reactive T cells, induction of T cell anergy or differentiation of antigen-specific Tregs (2). These immature DCs have been reported to be the primary cell types involved in maintaining tolerance in the periphery and mainly carry self-antigens. However, recent identification of partial- or semi-mature DCs with tolerizing capacities, questions the dogma that only immature DCs induce tolerance (22, 27). Similar to immunogenic DCs, tolDCs may be defined by integration of all the signals they transmit to T cells, including maturation marker expression, as well as the presence of, in this case, anti-inflammatory-related tolerizing signals consisting of surface molecule expression (PD-L1, ILT3/4, ICOSL, CTLA-4), tolerogenic cytokine profiles (IL-10, TGFβ) and the presence of other tolerance-inducing metabolites (IDO, RA) (14, 22). Furthermore, the presence or absence of pro-inflammatory cytokines seems to be decisive in inducing either immunity or tolerance, respectively. Nevertheless, no standard tolDCs profile has been established yet, and may not exist due to the great diversity between those that have been described till date.
42. Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. (2010) 59:595–604. doi: 10.1136/gut.2009.185108
71. Wadwa M, Klopfleisch R, Buer J, Westendorf AM. Targeting antigens to DEC-205 on dendritic cells induces immune protection in experimental colitis in mice. Eur J Microbiol Immunol. (2016) 6:1–8. doi: 10.1556/1886.2015.00048
Dendritic cells (DCs) are well-established as major players in the regulation of immune responses. They either induce inflammatory or tolerogenic responses, depending on the DC-subtype and stimuli they receive from the local environment. This dual capacity of DCs has raised therapeutic interest for their use to modify immune-activation via the generation of tolerogenic DCs (tolDCs). Several compounds such as vitamin D3, retinoic acid, dexamethasone, or IL-10 and TGF-β have shown potency in the induction of tolDCs. However, an increasing interest exists in defining tolerance inducing receptors on DCs for new targeting strategies aimed to develop tolerance inducing immunotherapies, on which we focus particular in this review. Ligation of specific cell surface molecules on DCs can result in antigen presentation to T cells in the presence of inhibitory costimulatory molecules and tolerogenic cytokines, giving rise to regulatory T cells. The combination of factors such as antigen structure and conformation, delivery method, and receptor specificity is of paramount importance. During the last decades, research provided many tools that can specifically target various receptors on DCs to induce a tolerogenic phenotype. Based on advances in the knowledge of pathogen recognition receptor expression profiles in human DC subsets, the most promising cell surface receptors that are currently being explored as possible targets for the induction of tolerance in DCs will be discussed. We also review the different strategies that are being tested to target DC receptors such as antigen-carbohydrate conjugates, antibody-antigen fusion proteins and antigen-adjuvant conjugates.
21. Busold S, Nagy NA, Tas SW, van Ree R, de Jong EC, Geijtenbeek TBH. Various tastes of sugar: the potential of glycosylation in targeting and modulating human immunity via C-type lectin receptors. Front Immunol. (2020) 11:1–12. doi: 10.3389/fimmu.2020.00134
102. Linehan SA, Martínez-Pomares L, Stahl PD, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: in situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. (1999) 189:1961–72. doi: 10.1084/jem.189.12.1961
125. Jenner J, Kerst G, Handgretinger R, Müller I. Increased α2,6-sialylation of surface proteins on tolerogenic, immature dendritic cells and regulatory T cells. Exp Hematol. (2006) 34:1211–7. doi: 10.1016/j.exphem.2006.04.016
There are two types of scanning probe microscope: the scanning tunneling microscope (STM) and the atomic force microscope (AFM). An STM uses a probe that is passed just above the specimen as a constant voltage bias creates the potential for an electric current between the probe and the specimen. This current occurs via quantum tunneling of electrons between the probe and the specimen, and the intensity of the current is dependent upon the distance between the probe and the specimen. The probe is moved horizontally above the surface and the intensity of the current is measured. Scanning tunneling microscopy can effectively map the structure of surfaces at a resolution at which individual atoms can be detected.
30. West HC, Bennett CL. Redefining the role of langerhans cells as immune regulators within the skin. Front Immunol. (2018) 8:1941. doi: 10.3389/fimmu.2017.01941
117. Petzold C, Schallenberg S, Stern JNH, Kretschmer K. Targeted antigen delivery to DEC-205+ dendritic cells for tolerogenic vaccination. Rev Diabet Stud. (2012) 9:305–18. doi: 10.1900/RDS.2012.9.305
Interestingly, this immune modulating mechanism has been exploited by pathogen and cancer cells. For example, the protozoan parasite Trypanosoma cruzi enzymatically cleaves sialic acid moieties from the host and transfers them via α2,3-linkage to its own surface, subsequently downregulating pro-inflammatory IL-12 production and upregulating anti-inflammatory IL-10 production in murine DCs (126). Furthermore, various tumors upregulate α2,3; α2,6; and α2,8 sialic acid on their surface to evade anti-tumor T cell responses and to induce tumor-specific tolerance (59). Comparable to these natural Siglec-mediated immune modulation events, the tolerance inducing capacity of Siglecs could be used in therapeutic strategies to treat auto-immune and allergic diseases. So far, several in vitro and in vivo mouse studies have been performed to address this concept (Table 1). Targeting Siglec H on murine pDCs using anti-Siglec-H-antigen (OVA or MOG peptides) conjugates resulted in a decrease of CD4+ T cell expansion and Th1/Th17 differentiation, which subsequently delayed the onset and reduced disease severity in EAE when using the anti-Siglec-H-MOG conjugate (79). Similarly, direct modification of OVA and MOG peptides with α2,3 or α2,6 sialyl-lactose targeted these antigens to Siglec E on DCs and dampened pro-inflammatory responses in the same EAE mouse model upon treatment with sialylated MOG peptides (80). Siglec E targeting resulted in the induction of Foxp3+ CD4+ Tregs and inhibition of inflammatory effector cells after stimulation with LPS, both in vitro and in vivo (80) (Figure 2). Finally, the potential of sialic acid modified antigens was tested in an experimental murine model for grass pollen allergy. Subcutaneous treatment with sialic acid modified grass pollen peptides induced significant numbers of antigen specific Tregs, inhibited antigen specific effect Th2 cells, and reduced the accumulation of eosinophils (81).
In IFA, secondary antibodies are stained with a fluorochrome rather than primary antibodies. Secondary antibodies do not attach directly to the pathogen, but they do bind to primary antibodies. When the unstained primary antibodies bind to the pathogen, the fluorescent secondary antibodies can be observed binding to the primary antibodies. Thus, the secondary antibodies are attached indirectly to the pathogen. Since multiple secondary antibodies can often attach to a primary antibody, IFA increases the number of fluorescent antibodies attached to the specimen, making it easier visualize features in the specimen (Figure \(\PageIndex{8}\)).
Many types of microscopes fall under the category of light microscopes, which use light to visualize images. Examples of light microscopes include brightfield microscopes, darkfield microscopes, phase-contrast microscopes, differential interference contrast microscopes, fluorescence microscopes, confocal scanning laser microscopes, and two-photon microscopes. These various types of light microscopes can be used to complement each other in diagnostics and research.
76. Sirvent S, Soria I, Cirauqui C, Cases B, Manzano AI, Diez-Rivero CM, et al. Novel vaccines targeting dendritic cells by coupling allergoids to nonoxidized mannan enhance allergen uptake and induce functional regulatory T cells through programmed death ligand 1. J Allergy Clin Immunol. (2016) 138:558–67.e11. doi: 10.1016/j.jaci.2016.02.029
115. Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, et al. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. (2008) 180:3647–50. doi: 10.4049/jimmunol.180.6.3647
3. Fucikova J, Palova-Jelinkova L, Bartunkova J, Spisek R. Induction of tolerance and immunity by dendritic cells: Mechanisms and clinical applications. Front Immunol. (2019) 10:2393. doi: 10.3389/fimmu.2019.02393
202296 — A bright enough light also makes it easier to blend colors and create a natural-looking contour. If you want your makeup to look its best, make ...
123. Hemmi H, Idoyaga J, Suda K, Suda N, Kennedy K, Noda M, et al. A new triggering receptor expressed on myeloid cells (trem) family member, trem-like 4, binds to dead cells and is a DNAX activation protein 12-linked marker for subsets of mouse macrophages and dendritic cells. J Immunol. (2009) 182:1278–86. doi: 10.4049/jimmunol.182.3.1278
57. Pereira MS, Alves I, Vicente M, Campar A, Silva MC, Padrão NA, et al. Glycans as key checkpoints of T cell activity and function. Front Immunol. (2018) 9:2754. doi: 10.3389/fimmu.2018.02754
20. Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. (2015) 15:471–85. doi: 10.1038/nri3865
22. Iberg CA, Hawiger D. Natural and induced tolerogenic dendritic cells. J Immunol. (2020) 204:733–44. doi: 10.4049/jimmunol.1901121
The maximum theoretical resolution of images created by light microscopes is ultimately limited by the wavelengths of visible light. Most light microscopes can only magnify 1000⨯, and a few can magnify up to 1500⨯, but this does not begin to approach the magnifying power of an electron microscope (EM), which uses short-wavelength electron beams rather than light to increase magnification and resolution.
66. Ring S, Maas M, Nettelbeck DM, Enk AH, Mahnke K. Targeting of autoantigens to DEC205 + dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J Immunol. (2013) 191:2938–47. doi: 10.4049/jimmunol.1202592
Table 1. Summary of in vivo studies to induce tolDCs using either antigen-antibody fusion compounds or carbohydrate-modified antigens.
A biofilm is a complex community of one or more microorganism species, typically forming as a slimy coating attached to a surface because of the production of an extrapolymeric substance (EPS) that attaches to a surface or at the interface between surfaces (e.g., between air and water). In nature, biofilms are abundant and frequently occupy complex niches within ecosystems (Figure \(\PageIndex{14}\)). In medicine,biofilms can coat medical devices and exist within the body. Because they possess unique characteristics, such as increased resistance against the immune system and to antimicrobial drugs, biofilms are of particular interest to microbiologists and clinicians alike.
110. Soria I, López-Relaño J, Viñuela M, Tudela JI, Angelina A, Benito-Villalvilla C, et al. Oral myeloid cells uptake allergoids coupled to mannan driving Th1/Treg responses upon sublingual delivery in mice. Allergy Eur J Allergy Clin Immunol. (2018) 73:875–84. doi: 10.1111/all.13396
34. Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes LA, et al. Functional specializations of human epidermal langerhans cells and CD14+ dermal dendritic cells. Immunity. (2008) 29:497–510. doi: 10.1016/j.immuni.2008.07.013
124. Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. (2007) 446:1023–9. doi: 10.1038/nature05816
94. Rodríguez E, Carasi P, Frigerio S, da Costa V, van Vliet S, Noya V, et al. Fasciola hepatica immune regulates CD11c+ cells by interacting with the macrophage gal/GalNAc lectin. Front Immunol. (2017) 8:264. doi: 10.3389/fimmu.2017.00264
The MR recognizes terminal mannose, fucose and N-acetylglycosamine carbohydrates via its carbohydrate recognition domains. In humans, the MR has been identified in CD1ahigh and CD1alow dermal DCs, as well as in vitro monocyte-derived DCs and macrophages (101). In mice, the MR is mainly expressed by tissue and lymphoid-resident macrophages, but also in various endothelial cells and tracheal smooth muscle cells (101, 102). Additionally, MR expression can be detected in cultured murine moDCs, however the in vivo expression of MR on murine DCs remains unknown (61, 101). The MR has been reported to induce DC-mediated anti-inflammatory responses, including IL-10 production upon binding to some natural ligands that bind inside the MR binding sites (103). In contrast, when MR interacts with ligands that bind outside the carbohydrate recognition domains, there is no induction of IL-10 secretion, suggesting that, the efficacy of MR-targeted vaccines to induce tolerance will greatly depend on the appropriate selection of targeting vehicles and conditions (104). Notably, in a murine autoimmune model of collagen antibody-induced arthritis, treatment of mice with an epitope of Leishmania analog of the receptors for activated C kinase (LACK) from Leishmania major, inhibited joint inflammation and downregulated Th1 and Th17 cell responses through binding to the MR in CD11c+ DCs (105). Similarly, mannosylated forms of the myelin peptide PLP139−151 and MOG induced a state of tolerance in EAE mice (74, 75). Inhibition of EAE disease severity was suggested to be mediated by modulation of peripheral autoreactive T cells. This is in agreement with a study where treatment with mannosylated OVA peptides induced impaired Th1 effector functions and abrogated the activity of pre-existing effector T cells (106).
91. Johannssen T, Lepenies B. Glycan-based cell targeting to modulate immune responses. Trends Biotechnol. (2017) 35:334–46. doi: 10.1016/j.tibtech.2016.10.002
When images are magnified, they become dimmer because there is less light per unit area of image. Highly magnified images produced by microscopes, therefore, require intense lighting. In a brightfield microscope, this light is provided by an illuminator, which is typically a high-intensity bulb below the stage. Light from the illuminator passes up through condenser lens (located below the stage), which focuses all of the light rays on the specimen to maximize illumination. The position of the condenser can be optimized using the attached condenser focus knob; once the optimal distance is established, the condenser should not be moved to adjust the brightness. If less-than-maximal light levels are needed, the amount of light striking the specimen can be easily adjusted by opening or closing a diaphragm between the condenser and the specimen. In some cases, brightness can also be adjusted using the rheostat, a dimmer switch that controls the intensity of the illuminator.
67. Stern JNH, Keskin DB, Kato Z, Waldner H, Schallenberg S, Anderson A, et al. Promoting tolerance to proteolipid protein-induced experimental autoimmune encephalomyelitis through targeting dendritic cells. Proc Natl Acad Sci USA. (2010) 107:17280–5. doi: 10.1073/pnas.1010263107
At very high magnifications, resolution may be compromised when light passes through the small amount of air between the specimen and the lens. This is due to the large difference between the refractive indices of air and glass; the air scatters the light rays before they can be focused by the lens. To solve this problem, a drop of oil can be used to fill the space between the specimen and an oil immersion lens, a special lens designed to be used with immersion oils. Since the oil has a refractive index very similar to that of glass, it increases the maximum angle at which light leaving the specimen can strike the lens. This increases the light collected and, thus, the resolution of the image (Figure \(\PageIndex{2}\)). A variety of oils can be used for different types of light.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
It is now recognized that DCs are a heterogeneous population of cells. The different subsets are defined by surface markers and transcriptome profiles, nicely reviewed by various colleagues (4, 13–16). DCs are generally classified into four major subsets, namely, CD141+ conventional DCs (cDC1s), CD1c+ conventional DCs (cDC2s), monocyte-derived DCs (moDCs), and plasmacytoid DCs (pDCs). The cDC1 subset is a relatively homogenous population that is specialized in cross-presentation of extracellular antigens and efficiently primes CD8+ T cells (16). In contrast, the cDC2 subset is a heterogeneous population and could be further subdivided in separate lineages. For example, the cDC2A and cDC2B lineage are defined by distinct developmental pathways regulated by the transcription factors T- bet and RORγt, respectively (17). Both lineages are potent stimulators of CD4+ naïve T cell, however, cDC2Bs have been shown to be more prone to secrete pro-inflammatory cytokine than cDC2As (13, 17). Additionally identified cDC2 lineages include monocyte-like DC2s, inducing Th1 responses, and DC3s, responsible for Th2, Th17 and Treg differentiation (15, 17). The moDC subset arises from monocytes and retains, like the DC3s, the monocyte marker CD14. They are recruited to inflamed tissue sites in vivo where they efficiently cross-present antigens to CD8+ T cells in peripheral tissues (18). The last subset, pDCs, differs from the other subsets as they are marked by quick secretion of pro-inflammatory type I interferons (IFN) following viral infection. pDCs are defined as CD123+ CD303+ CD304+ cells and were originally classified within the myeloid compartment. However, recent findings providing evidence for a lymphoid origin of the majority of pDCs challenges this hypothesis (4, 13, 19, 20). Finally, the tissues where DCs reside, such as lymph nodes, skin, lung, intestines and liver, offer the above mentioned DC subsets additional environmental factors to further adapt to their specific niche resulting in tissue specific DC subsets (8, 21, 22).
While the original fluorescent and confocal microscopes allowed better visualization of unique features in specimens, there were still problems that prevented optimum visualization. The effective sensitivity of fluorescence microscopy when viewing thick specimens was generally limited by out-of-focus flare, which resulted in poor resolution. This limitation was greatly reduced in the confocal microscope through the use of a confocal pinhole to reject out-of-focus background fluorescence with thin (<1 μm), unblurred optical sections. However, even the confocal microscopes lacked the resolution needed for viewing thick tissue samples. These problems were resolved with the development of the two-photon microscope, which uses a scanning technique, fluorochromes, and long-wavelength light (such as infrared) to visualize specimens. The low energy associated with the long-wavelength light means that two photons must strike a location at the same time to excite the fluorochrome. The low energy of the excitation light is less damaging to cells, and the long wavelength of the excitation light more easily penetrates deep into thick specimens. This makes the two-photon microscope useful for examining living cells within intact tissues—brain slices, embryos, whole organs, and even entire animals.
90. Li RE, van Vliet SJ, van Kooyk Y. Using the glycan toolbox for pathogenic interventions and glycan immunotherapy. Curr Opin Biotechnol. (2018) 51:24–31. doi: 10.1016/j.copbio.2017.11.003
118. Flacher V, Tripp CH, Mairhofer DG, Steinman RM, Stoitzner P, Idoyaga J, et al. Murine langerin + dermal dendritic cells prime CD 8 + T cells while L angerhans cells induce cross-tolerance. EMBO Mol Med. (2014) 6:1191–204. doi: 10.15252/emmm.201303283
41. Yamazaki S, Morita A. Dendritic cells in the periphery control antigen-specific natural and induced reulatory T cells. Front Immunol. (2013) 4:e151. doi: 10.3389/fimmu.2013.00151
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Use of a darkfield microscope allows us to view living, unstained samples of the spirochete Treponema pallidum. Similar to a photographic negative, the spirochetes appear bright against a dark background. (credit: Centers for Disease Control and Prevention/C.W. Hubbard)
15. Amon L, Lehmann CHK, Heger L, Heidkamp GF, Dudziak D. The ontogenetic path of human dendritic cells. Mol Immunol. (2020) 120:122–9. doi: 10.1016/j.molimm.2020.02.010
128. Seno A, Maruhashi T, Kaifu T, Yabe R, Fujikado N, Ma G, et al. Exacerbation of experimental autoimmune encephalomyelitis in mice deficient for DCIR, an inhibitory C-type lectin receptor. Exp Anim. (2015) 64:109–19. doi: 10.1538/expanim.14-0079
18. Schlitzer A, McGovern N, Ginhoux F. Dendritic cells and monocyte-derived cells: two complementary and integrated functional systems. Semin Cell Dev Biol. (2015) 41:9–22. doi: 10.1016/j.semcdb.2015.03.011
132. Tanriver Y, Ratnasothy K, Bucy RP, Lombardi G, Lechler R. Targeting MHC class I monomers to dendritic cells inhibits the indirect pathway of allorecognition and the production of IgG alloantibodies leading to long-term allograft survival. J Immunol. (2010) 184:1757–64. doi: 10.4049/jimmunol.0902987
Electrons, like electromagnetic radiation, can behave as waves, but with wavelengths of 0.005 nm, they can produce much better resolution than visible light. An EM can produce a sharp image that is magnified up to 100,000⨯. Thus, EMs can resolve subcellular structures as well as some molecular structures (e.g., single strands of DNA); however, electron microscopy cannot be used on living material because of the methods needed to prepare the specimens.
83. Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. (2003) 101:4862–9. doi: 10.1182/blood-2002-10-3229
44. del Fresno C, Iborra S, Saz-Leal P, Martínez-López M, Sancho D. Flexible signaling of Myeloid C-type lectin receptors in immunity and inflammation. Front Immunol. (2018) 9:1. doi: 10.3389/fimmu.2018.00804
6. Navarro-Barriuso J, Mansilla MJ, Naranjo-Gómez M, Sánchez-Pla A, Quirant-Sánchez B, Teniente-Serra A, et al. Comparative transcriptomic profile of tolerogenic dendritic cells differentiated with vitamin D3, dexamethasone and rapamycin. Sci Rep. (2018) 8:14985. doi: 10.1038/s41598-018-33248-7
16. Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. (2017) 356:eaah4573. doi: 10.1126/science.aah4573
45. Geijtenbeek TBH, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. (2009) 9:465–79. doi: 10.1038/nri2569
Electron microscopy can be used to observe biofilms, but only after dehydrating the specimen, which produces undesirable artifacts and distorts the specimen. In addition to these approaches, it is possible to follow water currents through the shapes (such as cones and mushrooms) of biofilms, using video of the movement of fluorescently coated beads (Figure \(\PageIndex{15}\)).
The human myeloid inhibitory C-type lectin receptor (MICL) or Clec12A is expressed on alveolar macrophages, cDC1s, cDC2s, and pDCs, while the mouse Clec12A is expressed on myeloid cells (45, 134). Clec12A selectively binds to dead cells that have lost their plasma membrane integrity (130). The endocytic capacity of Clec12A has led to its being exploited for DC-specific antigen targeting. In this regard, antibody-mediated targeting of OVA to Clec12A in mice was able to induce potent antibody responses but no tolerogenic responses (135). Such targeting of Clec12A with anti-Clec12A antibodies seems to be sufficient for antigen internalization, processing and presentation but not for activation of DCs as reported in targeting of DEC205 with mAbs (122, 135). These mAbs may therefore simply serve to deliver Ags to DCs. The study of Clec12A in the context of immunotherapy for dysregulated immune pathologies is still in its infancy and further research is warranted given that preliminary data and the biological properties of Clec12A portrays this receptor as a promising candidate in this field.
Figure 1. Ex vivo and in vivo strategies for generation of tolerogenic DCs for DC-based therapies. The current DC-based immunotherapy strategy in the treatment of immunopathologies involves the isolation of DC precursors either from PBMCs or bone marrow-derived cells which could either be allogeneic or autologous. These DC precursors are then differentiated into immature DCs in the presence of GM-CSF and recombinant IL-4 which are subsequently differentiated into tolerogenic DCs (tolDCs) by the addition of pharmacologic agents or immunomodulatory cytokines. Administration of these tolDCs leads to the generation of a suppressive immune environment which dampens inflammation. Future strategies are focusing more on in vivo targeting of DCs, where specific antigen-based vaccine formulations targeting specific receptors on DCs in their natural environment are injected into the patient. The antigen is taken up by DCs through these receptors, resulting in the induction of a tolerogenic program in DCs that leads to the generation of antigen-specific immunosuppression. DC, dendritic cells; GM-CSF, Granulocyte-macrophage colony-stimulating factor; IL-4, Interleukin 4; IL-10, Interleukin-10; TGF-β, Transforming growth factor beta.
Confocalmicroscopy
87. Inaba K, Swiggard WJ, Inaba M, Meltzer J, Miryza A, Sasagawa T, et al. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell Immunol. (1995) 163:148–56. doi: 10.1006/cimm.1995.1109
The LibreTexts libraries are Powered by NICE CXone Expert and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Legal. Accessibility Statement For more information contact us at info@libretexts.org.
108. Ilarregui JM, Rabinovich GA. Tolerogenic dendritic cells in the control of autoimmune neuroinflammation: an emerging role of protein-glycan interactions. Neuroimmunomodulation. (2010) 17:157–60. doi: 10.1159/000258712
28. Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. (2003) 21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040
Whereas other forms of light microscopy create an image that is maximally focused at a single distance from the observer (the depth, or z-plane), a confocal microscope uses a laser to scan multiple z-planes successively. This produces numerous two-dimensional, high-resolution images at various depths, which can be constructed into a three-dimensional image by a computer. As with fluorescence microscopes, fluorescent stains are generally used to increase contrast and resolution. Image clarity is further enhanced by a narrow aperture that eliminates any light that is not from the z-plane. Confocal microscopes are thus very useful for examining thick specimens such as biofilms, which can be examined alive and unfixed (Figure \(\PageIndex{9}\)).
Due to the unique capacity of DCs to coordinate innate and adaptive immune responses, they have been extensively studied and have proven to be a very promising strategy for immunotherapy. In the past decades, our knowledge of the potential of DCs in cancer and autoimmune disease/allergy therapy has expanded remarkably, advancing from the current ex vivo generated DC-based vaccines to in vivo targeting of DCs via specific receptors (Figure 1). Various compounds, such as vitamin D3, retinoic acid, dexamethasone, or IL-10, and TGFβ have shown the potency of tolDCs as immunotherapy in autoimmune diseases. However, there has been an increasing interest in moving toward in vivo targeting strategies where the induction of tolerance is achieved by targeting different receptors on DCs in their natural environment with antigen-delivering antibodies and antigen-carbohydrate conjugates. This has proven to be very effective in the amelioration of disease processes in a range of mouse models including MS, diabetes and allergies. Nevertheless, there is still a need to expand our knowledge on the potential application of such in vivo targeting strategies in human settings because, despite the many important similarities that exist between human and mouse DCs, very crucial incompatibilities between both species still limits the capacity to translate findings from one species to the other. Nonetheless, the potential for future clinical translation and therapeutic application of in vivo antigen targeting to DCs is very promising, although additional research is necessary to decipher the specific molecular mechanisms involved in the anti-disease tolerance promoted by such DCs. During the development of potential vaccines for autoimmune and allergic diseases, multiple inevitable questions need to be addressed. For instance, receptors that are not inherently tolerogenic but are capable of inducing tolerance under certain conditions, such as DEC205, DC-SIGN, and langerin, the induced tolerogenic effect is abrogated in the presence of pro-inflammatory modulators. Therefore, the appropriate optimization of vaccine formulations to target such receptors will be of utmost importance. In contrast, Siglecs have the ability to induce tolerogenic immune responses even in the presence of the pro-inflammatory modulator LPS (80, 136) and the resulting responses are not particularly affected by the presence of adjuvants. Moreover, there is still uncertainty about the right antigen-antibody/glycan dosage necessary for induction of tolerance, the duration of the resulting tolerogenic response, the effect on other immune cells expressing similar receptors as those being targeted, the use of a vehicle, and the method of administration. Finally, it may also be important to further investigate the potential positive or negative effects that receptor-specific antigen targeting may have on other myeloid cells, such as macrophages that express some of the DC receptors that can be targeted. Although further investigation is warranted, the effects might be negligible giving the lower antigen presenting capacity of macrophages. Overall, it is clear that the generation of a tolerogenic immune response via DC receptor targeting depends on the receptor, the DC subset being targeted, and the specific micro-environmental factors.
13. Rhodes JW, Tong O, Harman AN, Turville SG. Human dendritic cell subsets, ontogeny, and impact on HIV infection. Front Immunol. (2019) 10:1088. doi: 10.3389/fimmu.2019.01088
26. Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. (2012) 30:1–22. doi: 10.1146/annurev-immunol-100311-102839
CC and B-CKD performed the literature search, wrote the manuscript, and created all figures. EdJ and YvK critically read and carefully revised all versions of the manuscript providing valuable guidance and insight. RvR critically read the manuscript and provided valuable additions. All authors contributed to the article and approved the submitted version.
32. Devi KSP, Anandasabapathy N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin Immunopathol. (2017) 39:137–52. doi: 10.1007/s00281-016-0602-0
DCs are able to recognize and take up DAMPs through surface receptors such as Clec9A, a homodimeric type II transmembrane protein with a single extracellular C-type lectin-like domain expressed on cDC1s in both mice and human (119–121). The highly restricted expression of Clec9A on the human and mice cDC1 subset makes it an attractive receptor for targeting this specific subset of DCs (118). Clec9A ligation to its ligand F-actin either results in immunity or tolerance. As Clec9A promotes CD8+ T cell cross-priming, several in vitro studies have been performed to explore Clec9A targeting to induce anti-tumor immune responses (121). To determine whether Clec9A is a promising receptor for DC targeting in the context of autoimmune diseases, mice were injected with anti-Clec9A-antigen conjugates. In steady-state conditions in the absence of adjuvants, these conjugates promoted the differentiation of Foxp3+ Treg cells (62) (Figure 2). On the other hand, when anti-Clec9A was administered in combination with polyI:C, tolerance was prevented and instead promoted the development of potent antibody and Th1 or Th17 responses (62). Also, it has been reported that antigen delivery via Clec9A enhances the humoral response, even in the absence of adjuvant CpG. However, the immunoglobulin classes and resulting tolerogenic or immunogenic functions, were not explored in this study (122). Although targeting Clec9A can induce Tregs, extensive research is still needed to perfectly map out the optimal conditions that are necessary for tolerance induction.
37. Comi M, Amodio G, Gregori S. Interleukin-10-producing DC-10 is a unique tool to promote tolerance via antigen-specific T regulatory type 1 cells. Front Immunol. (2018) 9:6. doi: 10.3389/fimmu.2018.00682
38. Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. (2010) 116:935–44. doi: 10.1182/blood-2009-07-234872
58. Crespo HJ, Lau JTY, Videira PA. Dendritic cells: a spot on sialic acid. Front Immunol. (2013) 4:491. doi: 10.3389/fimmu.2013.00491
33. Chu CC, Ali N, Karagiannis P, Di Meglio P, Skowera A, Napolitano L, et al. Resident CD141 (BDCA3) + dendritic cells in human skin produce IL-10 and induce regulatory T cells that suppress skin inflammation. J Exp Med. (2012) 209:935–45. doi: 10.1084/jem.20112583
9. Ten Brinke A, Martinez-Llordella M, Cools N, Hilkens CMU, Van Ham SM, Sawitzki B, et al. Ways forward for tolerance-inducing cellular therapies- An afactt perspective. Front Immunol. (2019) 10:181. doi: 10.3389/fimmu.2019.00181
68. Spiering R, Margry B, Keijzer C, Petzold C, Hoek A, Wagenaar-Hilbers J, et al. DEC205 + dendritic cell–targeted tolerogenic vaccination promotes immune tolerance in experimental autoimmune arthritis. J Immunol. (2015) 194:4804–13. doi: 10.4049/jimmunol.1400986
62. Joffre OP, Sancho D, Zelenay S, Keller AM, Reis E, Sousa C. Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur J Immunol. (2010) 40:1255–65. doi: 10.1002/eji.201040419
78. Mathiesen CBK, Carlsson MC, Brand S, Möller SR, Idorn M, Thor Straten P, et al. Genetically engineered cell factories produce glycoengineered vaccines that target antigen-presenting cells and reduce antigen-specific T-cell reactivity. J Allergy Clin Immunol. (2018) 142:1983–7. doi: 10.1016/j.jaci.2018.07.030
79. Loschko J, Heink S, Hackl D, Dudziak D, Reindl W, Korn T, et al. Antigen targeting to plasmacytoid dendritic cells via siglec-h inhibits Th cell-dependent autoimmunity. J Immunol. (2011) 187:6346–56. doi: 10.4049/jimmunol.1102307
69. Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y, et al. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes. (2005) 54:3395–401. doi: 10.2337/diabetes.54.12.3395
86. Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. (2004) 20:695–705. doi: 10.1016/j.immuni.2004.05.002
Because biofilms are thick, they cannot be observed very well using light microscopy; slicing a biofilm to create a thinner specimen might kill or disturb the microbial community. Confocal microscopy provides clearer images of biofilms because it can focus on one z-plane at a time and produce a three-dimensional image of a thick specimen. Fluorescent dyes can be helpful in identifying cells within the matrix. Additionally, techniques such as immunofluorescence and fluorescence in situ hybridization (FISH), in which fluorescent probes are used to bind to DNA, can be used.
122. Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee C-N, et al. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J Immunol. (2011) 187:842–50. doi: 10.4049/jimmunol.1101176
74. Kel J, Oldenampsen J, Luca M, Drijfhout JW, Koning F, Nagelkerken L. Soluble mannosylated myelin peptide inhibits the encephalitogenicity of autoreactive T cells during experimental autoimmune encephalomyelitis. Am J Pathol. (2007) 170:272–80. doi: 10.2353/ajpath.2007.060335
For electrons to pass through the specimen in a TEM, the specimen must be extremely thin (20–100 nm thick). The image is produced because of varying opacity in various parts of the specimen. This opacity can be enhanced by staining the specimen with materials such as heavy metals, which are electron dense. TEM requires that the beam and specimen be in a vacuum and that the specimen be very thin and dehydrated. The specific steps needed to prepare a specimen for observation under an EM are discussed in detail in the next section.
81. Hesse L, Feenstra R, Ambrosini M, de Jager WA, Petersen A, Vietor H, et al. Subcutaneous immunotherapy using modified Phl p5a-derived peptides efficiently alleviates allergic asthma in mice. Allergy Eur J Allergy Clin Immunol. (2019) 74:2495–8. doi: 10.1111/all.13918
Treml4 is another cell death receptor and binds to late apoptotic bodies necrotic cells (123). It is a member of the the triggering receptor expressed on myeloid cells (Trem)-family receptors which are primarily expressed on murine CD8+ lymphoid resident DCs and CD103+ lung DCs (63, 123). Treml4 has been investigated as a therapeutic target in a study by Idoyaga and colleagues. In this study, it was demonstrated that, intranasal inoculation of anti-Treml4-MOG peptide conjugates could induce MOG-specific Foxp3+ T cells in mice, but this did not prevent the development or promote improvement of EAE symptoms in diseased mice (63). The molecular mechanisms underlying these observations were not explored but it seems like signaling through this receptor does not produce a strong enough signal necessary for DC-mediated polarization of T cells to Tregs or the suppressive capacity of induced Tregs may be impaired in some way. Mechanistic studies addressing these issues will be very valuable in further exploring the therapeutic potential of this receptor in human settings in the context of immune pathologies. It is also important to note that the expression of Treml4 on human DC is yet to be reported. Therefore, the use of cell death sensing receptors for tolerizing therapies remains elusive until additional studies shed light on their relevance for clinical applications.
For example, if a \(40 \times\) objective lens is selected and the ocular lens is \(10\times\), the total magnification would be
A scanning probe microscope does not use light or electrons, but rather very sharp probes that are passed over the surface of the specimen and interact with it directly. This produces information that can be assembled into images with magnifications up to 100,000,000⨯. Such large magnifications can be used to observe individual atoms on surfaces. To date, these techniques have been used primarily for research rather than for diagnostics.
MGL is well-characterized for its specificity for terminal GalNAc (N-Acetylgalactosamine) residues, expressed by both mammalian cells and pathogens (49). In humans, MGL is expressed in vivo by human DCs of skin and lymph nodes and in vitro by macrophages and moDCs (107). In mice however, the homologs of human MGL, MGL1, and MGL2 are expressed by dermal DCs and alternatively activated macrophages. Upon ligand binding, the intracellular signaling pathways that are triggered vary extensively depending on the structure of the ligands. In this regard, it has recently been reported that, glycoconjugates from Fasciola hepatica potentiate the production of IL-10 by moDCs via engagement of MGL (94). Moreover, MGL-expressing DCs from mice infected with these glycoconjugates expanded IL-10-producing T cells and suppressed Th1 responses. Correspondingly, recent data has labeled the MGL as a negative regulator in autoimmune-induced neuroinflammation as MGL was shown to induce apoptosis of autoreactive T cells, the reduction of autoantibodies and the induction of IL-10 (108).
Fluorescence microscope
99. Maizels RM, Smits HH, McSorley HJ. Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity. (2018) 49:801–18. doi: 10.1016/j.immuni.2018.10.016
95. Klaver EJ, Kuijk LM, Laan LC, Kringel H, van Vliet SJ, Bouma G, et al. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int J Parasitol. (2013) 43:191–200. doi: 10.1016/j.ijpara.2012.10.021
Phase contrastmicroscopy
48. Iborra S, Sancho D. Signalling versatility following self and non-self sensing by myeloid C-type lectin receptors. Immunobiology. (2015) 220:175–84. doi: 10.1016/j.imbio.2014.09.013
135. Lahoud MH, Proietto AI, Ahmet F, Kitsoulis S, Eidsmo L, Wu L, et al. The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses. J Immunol. (2009) 182:7587–94. doi: 10.4049/jimmunol.0900464
72. Kamoi K, Martín-Granados C, Bobu C, Wikstrom M, Degli-Esposti M, Steinman R, et al. Anti-DEC205 mediated delivery of self-antigen to dendritic cell restores tolerance in spontaneous EAU. Invest Ophthalmol Vis Sci. (2012) 53:6233.
Even a very powerful microscope cannot deliver high-resolution images if it is not properly cleaned and maintained. Since lenses are carefully designed and manufactured to refract light with a high degree of precision, even a slightly dirty or scratched lens will refract light in unintended ways, degrading the image of the specimen. In addition, microscopes are rather delicate instruments, and great care must be taken to avoid damaging parts and surfaces. Among other things, proper care of a microscope includes the following:
47. García-Vallejo JJ, Van Kooyk Y. Endogenous ligands for C-type lectin receptors: the true regulators of immune homeostasis. Immunol Rev. (2009) 230:22–37. doi: 10.1111/j.1600-065X.2009.00786.x
Made to highlight specific objects or areas in a room, spotlights can be angled to shine bright light where you need it. With smart spotlights, you can get all ...
103. Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. (2003) 171:4552–60. doi: 10.4049/jimmunol.171.9.4552
82. Mahnke K, Ring S, Enk AH. Antibody targeting of “steady-state” dendritic cells induces tolerance mediated by regulatory T cells. Front Immunol. (2016) 7:63. doi: 10.3389/fimmu.2016.00063
Under the brightfield microscope, the technician can barely see the bacteria cells because they are nearly transparent against the bright background. To increase contrast, the technician inserts an opaque light stop above the illuminator. The resulting darkfield image clearly shows that the bacteria cells are spherical and grouped in clusters, like grapes.
A fluorescence microscope uses fluorescent chromophores called fluorochromes, which are capable of absorbing energy from a light source and then emitting this energy as visible light. Fluorochromes include naturally fluorescent substances (such as chlorophylls) as well as fluorescent stains that are added to the specimen to create contrast. Dyes such as Texas red and FITC are examples of fluorochromes. Other examples include the nucleic acid dyes 4’,6’-diamidino-2-phenylindole (DAPI) and acridine orange.
Differential interference contrast (DIC) microscopes (also known as Nomarski optics) are similar to phase-contrast microscopes in that they use interference patterns to enhance contrast between different features of a specimen. In a DIC microscope, two beams of light are created in which the direction of wave movement (polarization) differs. Once the beams pass through either the specimen or specimen-free space, they are recombined and effects of the specimens cause differences in the interference patterns generated by the combining of the beams. This results in high-contrast images of living organisms with a three-dimensional appearance. These microscopes are especially useful in distinguishing structures within live, unstained specimens. (Figure \(\PageIndex{7}\)).
130. Kaifu T, Iwakura Y. Dendritic cell immunoreceptor (DCIR): an ITIMharboring C-type lectin receptor. In: C-Type Lectin Receptors in Immunity. Springer Japan. (2016). p. 101–13. doi: 10.1007/978-4-431-56015-9_7
Langerin (CD207) is a transmembrane protein that functions as an endocytic receptor by binding various sugars, including mannose, n-acetylglucosamine, fucose, and sulfated sugars, and mediates efficient antigen presentation on MHC I and II products in vivo (115). Langerin is highly expressed on surfaces of human LCs, but also at low levels on cDC2s isolated from dermal, lung, liver and lymphoid tissue (116). Yet, langerin is not expressed on circulating cDC2s isolated from blood (116). In mice, langerin is expressed on LCs and CD8α+DEC205+ cDC1s of the spleen and skin draining, however, langerin has not been identified in the homologous human cDC1 subset, indicating that langerin targeting strategies could induce distinct outcomes between mice and human experiments due to differential expression of the targeting receptor in both species (44, 52). Nevertheless, the tolerogenic role of LCs under physiological conditions as well as their accessible location at the surface of the body, marked langerin as a promising target for in vivo delivery of self-antigens to alter disease severity in autoimmune diseases (30, 31, 63). A notable mention is a report from Idoyaga et al that showed that, targeting MOG35−55 peptides to murine skin and lung langerin+ migratory DCs via conjugation with anti-langerin antibodies lessens EAE symptom severity through the induction of Foxp3+ Tregs (63) (Figure 2). The effect was comparable to the reduction of disease symptoms following administration of anti-DEC205-MOG fusion proteins. Interestingly, the langerin+ DC population is known to co-express high levels of DEC205, suggesting that the DC subset that is targeted with anti-langerin is also a target for anti-DEC205 mediated induction of Foxp3+ Tregs (117). However, in contrast to the above findings, co-administration of recombinant langerin-mAbs fused to antigen and maturation stimuli like anti-CD40 or polyI:C leads to efficient CD4+ and CD8+ T cell priming, proliferation, and differentiation (118). These results suggest that the strength of the activation signal targeted to langerin+ DC is a very important factor that must be strictly controlled in order to exploit these subsets in autoimmune therapy. Nonetheless, the ability of langerin+ DCs to induce antigen-specific Foxp3+ Tregs in lungs, suggest that anti-langerin mAbs is an attractive candidate for the treatment of respiratory dysregulated immune responses like allergies (Figure 2).
KLM MINI led spotlights are small in size like cut hole only 18mm and can achieve precise lighting in small or complex spaces. Come with the 1-10V dimming ...
Siglec receptors are a family of receptors expressed on a wide variety of immune cells, including DCs (43, 59). They recognize sialic acid, which is the last carbohydrate structure added during the process of glycosylation, positioning sialic acid groups on the distal end of sugar-moieties (124). These sialic acid groups are present in the glycocalyx of all mammalian cells and could be considered as SAMPs (43). Siglecs are divided into two groups: (1) Siglecs that are conserved throughout different species, namely Siglec-1 (sialoadhesin), Siglec-2 (CD22), Siglec-4 myelin associated glycoprotein (MAG) and Siglec-15, and (2) the CD33-related Siglecs that have rapidly expanded and have no clear orthologs in mammalian species viz; Siglec−3 (CD33), −5, −6, −7, −8, −9, −10, −11, −14 and −16. The expression of Siglecs on myeoloid cells and their subsequent intracellular signaling pathways have been nicely reviewd by Lübbers et al. (59). In short, monocytes and monocyte-derived DCs express high levels of Siglec-3, −7 and −9, and low levels of Siglec-10. cDCs also express Siglec-3, −7, and −9, augmented with low expression levels of Siglec-2 and −15. On the other hand, pDCs only express Siglec-1, which is a non-signaling Siglec that internalizes upon ligand binding, and Siglec-5 (59). The binding affinity to sialic-acid-containing glycan varies between Siglecs. This is determined by the linkage of the sialic acid group to the underlying carbohydrate moiety (α2,3; α2,6 or α2,8 linkage). Another noteworthy feature of Siglecs is their ability to either bind their ligand via a trans interaction (on a different cell) or via a cis interaction (on the same cell). These cis interactions might contribute to sustain a tolerogenic phenotype, as surface proteins on tolerogenic DCs, immature DCs and Tregs are highly α2,6-sialylated, and could serve as a ligand for tolerogenic Siglecs (125). The conserved Siglec-2 and the CD33-related Siglec-3, and−5 till−11 contain an intracellular ITIM or ITIM-like motif that deliver negative signals via recruitment of SHP1 and SHP2 (43).
DEC205 (CD205) is an endocytic receptor highly expressed on cDC1s and belongs to the macrophage- mannose receptor family of CLRs (54). Although the natural ligand of DEC205 remains to be elucidated, some studies suggest apoptotic and necrotic material as well as CpG motifs as consecutive ligands (60). Upon antigen encounter, DEC205 internalizes and recycles very efficiently back to the surface (61). DEC205 was one of the first receptors used for in vivo antibody targeting of DCs (Table 1). Initial experiments using model antigens, such as hen egg lysozyme or ovalbumin (OVA), coupled to anti-DEC205 antibodies, demonstrated that these antigens were taken up by DCs (82–84). When OVA-anti-DEC205 fusion antibodies were administered to mice in the presence of maturation stimuli, strong immunogenic responses were induced (85). Conversely, when anti-DEC-antigens were injected into animals without adjuvants, DCs remained non-activated as their expression levels of costimulatory molecules was comparable to those obtained in DCs from control mice (83, 85). The analysis of antigen-specific T cell populations in injected mice revealed increased numbers of antigen-specific IL-10 producing CD25+Foxp3+ Tregs that were able to suppress proliferation of CD4+ T cells in vivo (83) (Figure 2). Since then, DEC205 targeting has been tested in various autoimmune disease animal models. For instance, in experimental autoimmune encephalomyelitis (EAE), the murine model for MS (63, 66, 86), injection of the autoantigen, myelin oligodendrocyte glycoprotein (MOG) or proteolipid protein (PLP) fused to anti-DEC205-specific antibodies led to elicitation of IL-10-producing CD4+CD25+Foxp3+ Tregs, the deletion of antigen-specific CD4+ and CD8+ T cells, reduced Th17 cell activity and significantly ameliorated disease symptoms and substantially delayed the disease onset (63, 64, 67) (Figure 2). In some studies, the conversion of some autoreactive T cells into Foxp3+ pTreg cells was reported. These findings were confirmed in non-obese diabetic (NOD), a mouse model of T1D, inflammatory bowel disease (IBD) (71), proteoglycan-arthritis (68), spontaneous experimental autoimmune uveoretinitis (EAU) (72), an animal model of ocular inflammation, as well as a model of graft-vs. host disease (65, 69, 70, 73). Because the use of DEC205 antibodies has proven to be more effective than the administration of free synthetic peptides, in these models of autoimmune diabetes, it is being considered as a possible, important therapeutic tool in the treatment of various autoimmune diseases (Table 1). In summary, these data establish DC targeting via DEC205 as an effective strategy to tolerize against autoantigens to protect against autoimmunity. However, this DC targeting strategy for the induction of tolerance is yet to be tested in human settings. Moreover, the future prospects of targeting DEC205 to induce tolerance in humans may be hampered by the varied expression pattern of this receptor on the different subsets of DCs (87). Although DEC-205 is predominantly expressed in mice CD8+ DCs, dermal DCs and LCs, human DEC-205 is relatively high expressed on myeloid blood DCs and monocytes, at moderate levels on B cells, and at low levels on pDCs, T cells and natural killer cells (87, 88). This differential expression pattern of DEC205 in humans is problematic for the development of DEC205-targeted vaccines for humans due to potential offsite targeting and needs to be addressed carefully during the design of potential clinical studies.
2. Audiger C, Rahman MJ, Yun TJ, Tarbell KV, Lesage S. The importance of dendritic cells in maintaining immune tolerance. J Immunol. (2017) 198:2223–31. doi: 10.4049/jimmunol.1601629
129. Fujikado N, Saijo S, Yonezawa T, Shimamori K, Ishii A, Sugai S, et al. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med. (2008) 14:176–80. doi: 10.1038/nm1697
107. van Vliet SJ, Paessens LC, Broks-van den Berg VCM, Geijtenbeek TBH, van Kooyk Y. The C-type lectin macrophage galactose-type lectin impedes migration of immature APCs. J Immunol. (2008) 181:3148–55. doi: 10.4049/jimmunol.181.5.3148
Dendritic cells (DCs) are important antigen presenting cells during the induction of immune responses and are essential in directing immune responses toward either immunity or tolerance. This decision is of great importance as undesired inflammatory responses could cause autoimmune or allergic diseases. In the periphery, DCs capture antigens and process them while migrating to the draining lymph nodes, where they present antigen-specific peptides to T lymphocytes. This migration process causes a dramatic transformation of the DC phenotype, called maturation. Maturation is associated with increased MHC-II complex levels, costimulatory molecule expression, enhanced secretion of polarizing cytokines and molecules, and alterations in chemokine receptor expression, all resulting in an optimal microenvironment to direct T cell responses (1–3). Although various DC subsets have been shown to preferentially induce specific T cell responses in non-inflammatory conditions, the induction of T cell immunity is adapted to and dictated by the encounter with pathogens (4). The unique capacity of DCs to coordinate innate and adaptive immune responses has highlighted them as potential targets for immune activating or dampening therapies to combat undesired immune responses (3).
43. Läubli H, Varki A. Sialic acid–binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses. Cell Mol Life Sci. (2020) 77:593–605. doi: 10.1007/s00018-019-03288-x
Overall, these studies demonstrate that targeting DC through Siglecs could be very promising for induction of tolerogenic immune responses as a treatment for autoimmune and allergic diseases (Figure 2). However, most studies have been performed in mice and are therefore not sufficient for translation to human settings. Consequently, it is important to elucidate their potential and consequences in the human immune system.
133. Iberg CA, Hawiger D. Advancing immunomodulation by in vivo antigen delivery to DEC-205 and other cell surface molecules using recombinant chimeric antibodies. Int Immunopharmacol. (2019) 73:575–80. doi: 10.1016/j.intimp.2019.05.037
29. Adnan E, Matsumoto T, Ishizaki J, Onishi S, Suemori K, Yasukawa M, et al. Human tolerogenic dendritic cells generated with protein kinase C inhibitor are optimal for functional regulatory T cell induction—A comparative study. Clin Immunol. (2016) 173:96–108. doi: 10.1016/j.clim.2016.09.007
Because it increases contrast without requiring stains, phase-contrast microscopy is often used to observe live specimens. Certain structures, such as organelles in eukaryotic cells and endospores in prokaryotic cells, are especially well visualized with phase-contrast microscopy (Figure \(\PageIndex{6}\)).
131. Meyer-Wentrup F, Cambi A, Joosten B, Looman MW, de Vries IJM, Figdor CG, et al. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J Leukoc Biol. (2009) 85:518–25. doi: 10.1189/jlb.0608352
After calling a local doctor about Cindy’s case, the camp nurse sends the sample from the wound to the closest medical laboratory. Unfortunately, since the camp is in a remote area, the nearest lab is small and poorly equipped. A more modern lab would likely use other methods to culture, grow, and identify the bacteria, but in this case, the technician decides to make a wet mount from the specimen and view it under a brightfield microscope. In a wet mount, a small drop of water is added to the slide, and a cover slip is placed over the specimen to keep it in place before it is positioned under the objective lens.
27. Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. (2002) 23:445–9. doi: 10.1016/S1471-4906(02)02281-0
1. Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. (2013) 31:563–604. doi: 10.1146/annurev-immunol-020711-074950
54. Streng-Ouwehand I, Ho NI, Litjens M, Kalay H, Boks MA, Cornelissen LAM, et al. Glycan modification of antigen alters its intracellular routing in dendritic cells, promoting priming of T cells. Elife. (2016) 5:11765. doi: 10.7554/eLife.11765
Next to CLRs, the Siglecs are also expressed on DCs and recognize self- and non-self-antigens (43, 46). Similar to CLRs, they could serve as adhesion molecules and endocytic receptors, and have been shown to be important instructors of T cell immunity (57, 58). Most members of the Siglec family signal through ITIM or ITIM-like motifs resulting in the generation of anti-inflammatory signals that modulate DC function (59). Overall, the ability of effective antigen uptake, processing, and presentation as well as the regulation of immunogenic and tolerogenic immune responses through the modulation of DC function positions DC receptors as promising candidates for novel DC-targeting immunotherapies. In the next sections, we explore current knowledge regarding the most promising DC-receptors being targeted for in vivo generation of tolDCs for the treatment of immune dysregulated pathologies.
92. Marciani DJ. Effects of immunomodulators on the response induced by vaccines against autoimmune diseases. Autoimmunity. (2017) 50:393–402. doi: 10.1080/08916934.2017.1373766
49. Zizzari IG, Napoletano C, Battisti F, Rahimi H, Caponnetto S, Pierelli L, et al. MGL receptor and immunity: when the ligand can make the difference. J Immunol Res. (2015) 2015:450695. doi: 10.1155/2015/450695
8. Obregon C, Kumar R, Pascual MA, Vassalli G, Golshayan D. Update on dendritic cell-induced immunological and clinical tolerance. Front Immunol. (2017) 8:1514. doi: 10.3389/fimmu.2017.01514
109. Benito-Villalvilla C, Soria I, Subiza JL, Palomares O. Novel vaccines targeting dendritic cells by coupling allergoids to mannan. Allergo J Int. (2018) 27:256–62. doi: 10.1007/s40629-018-0069-8
36. Amodio G, Mugione A, Sanchez AM, Viganò P, Candiani M, Somigliana E, et al. HLA-G expressing DC-10 and CD4+ T cells accumulate in human decidua during pregnancy. Hum Immunol. (2013) 74:406–11. doi: 10.1016/j.humimm.2012.11.031
Differential interference contrastmicroscopy
70. Mukhopadhaya A, Hanafusa T, Jarchum I, Chen YG, Iwai Y, Serreze DV, et al. Selective delivery of β cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc Natl Acad Sci USA. (2008) 105:6374–9. doi: 10.1073/pnas.0802644105
DCIR is another member of the family of CLRs expressed on cDCs, moDCs and pDCs (44, 45, 127). The human DCIR-Fc protein has been reported to bind a variety of carbohydrate structures including Lewisb, Man3 glycans, and bisecting GlcNAc residues (127). The mouse homolog of DCIR, DCIR2 is primarily expressed on CD8+DCs. DCIR is important for the homeostasis of the immune system by, in part, regulating DC differentiation or polarization, as DCIR-deficient mice were prone to develop autoimmune encephalitis (128). As such, consistent with results obtained in mouse models (128, 129), polymorphisms of the DCIR gene are associated with the susceptibility to RA in humans (130). DCIR2+ DCs have been shown to stimulate natural Foxp3+ Tregs to mediate tolerance to self-antigens in the absence of immune stimuli (41). However, in the presence of a maturation stimulus, they induce T cell expansion and production of pro-inflammatory cytokines (41). Upon triggering with DCIR-specific mAbs, DCIR is internalized in both pDCs as well as human moDCs, resulting in efficient antigen presentation to T cells and downregulation of IFN-α production (131). Interestingly, using an anti-DCIR2-PLP139−151 and MOG fusion antibody to target DCs resulted in the amelioration of EAE symptoms (63, 64). This effect was suggested to be mediated primarily through the depletion of autoreactive T cells or induction of anergy in pathogenic T cells (Figure 2). However, DCIR2-targeting did not induce de novo generation of Ag-specific Treg from naïve CD4+ T cell precursors in the steady state due to lack of TGF-β expression by CD4+ CD11b+ DC but instead stimulated and expanded natural Tregs (64). Similarly, targeting β-Cell antigen using chimeric DCIR2 antibodies in NOD mice, elicited tolerogenic CD4+ T cell responses and induced increased T cell apoptosis while delaying diabetes induction (65). Targeting DCs with anti-DCIR2-antigen has also shown some promise in the field of transplantation where, an anti-DCIR2-MHC I monomer successfully inhibited allorecognition and the production of IgG alloantibodies leading to long-term allograft survival (132). Unexpectedly, DCIR2 targeting of mice DCs augmented spontaneous EAU development, characterized by local reduction in Tregs (133). While DCIR2 may be a promising candidate for in vivo Ag delivery in mice, this may not be the case for humans because, even though DCIR2 is highly restricted to CD11b+ DC subset in mice it is not detectable on the human CD1a/b+ cDC subset, a proposed equivalent of mouse CD11b+ DCs (130). Thus, there is need for identification of surface molecules that are specifically and similarly expressed by mouse and human DCs to allow exploration of clinical effectiveness of vaccines targeting these DC subsets.
There are two basic types of EM: the transmission electron microscope (TEM) and the scanning electron microscope (SEM)(Figure \(\PageIndex{10}\)). The TEM is somewhat analogous to the brightfield light microscope in terms of the way it functions. However, it uses an electron beam from above the specimen that is focused using a magnetic lens (rather than a glass lens) and projected through the specimen onto a detector. Electrons pass through the specimen, and then the detector captures the image (Figure \(\PageIndex{11}\)).
CLRs function both as adhesion molecules and endocytic receptors, but also have a function in directing immunity to various pathogens, cellular proteins and lipids (44, 47, 48). Induction of immune response through these receptors can alternate between inflammation and immune tolerance depending on several factors including the nature of the ligand (49). They recognize a large and diverse range of ligands and trigger immune responses by inducing signaling pathways via an immunoreceptor tyrosine-based activation motif (ITAM), ITAM-like motif, or immunoreceptor tyrosine-based inhibitory motif (ITIM) that signal through Syk or phosphatases (21, 44, 45, 49, 50), generating pro- or anti-inflammatory signals, respectively. Only a few CLRs, such as, DC immunoreceptor (DCIR), Clec12A and Clec12B, bear the ITIM motif (51). Moreover, an important number of CLRs do not signal through Syk or phosphatases but may bear ITAM/ITIM motifs which are important for endocytosis (45, 52). Examples include: dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), LSECtin, macrophage C-type lectin (MCL), Langerin, macrophage-galactose lectin (MGL), mannose receptor (MR) and DEC205 (11, 44, 48). These CLRs mediate antigen internalization, followed by processing and subsequent presentation via MHC-I or II molecules (53–56).
80. Perdicchio M, Ilarregui JM, Verstege MI, Cornelissen LAM, Schetters STT, Engels S, et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc Natl Acad Sci USA. (2016) 113:3329–34. doi: 10.1073/pnas.1507706113
40. Tordesillas L, Berin MC. Mechanisms of oral tolerance. Clin Rev Allergy Immunol. (2018) 55:107–17. doi: 10.1007/s12016-018-8680-5
Darkfield microscopy can often create high-contrast, high-resolution images of specimens without the use of stains, which is particularly useful for viewing live specimens that might be killed or otherwise compromised by the stains. For example, thin spirochetes like Treponema pallidum, the causative agent of syphilis, can be best viewed using a darkfield microscope (Figure \(\PageIndex{4}\)).
55. Nair P, Amsen D, Blander JM. Co-ordination of incoming and outgoing traffic in antigen-presenting cells by pattern recognition receptors and T Cells. Traffic. (2011) 12:1669–76. doi: 10.1111/j.1600-0854.2011.01251.x
88. Kato M, McDonald KJ, Khan S, Ross IL, Vuckovic S, Chen K, et al. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int Immunol. (2006) 18:857–69. doi: 10.1093/intimm/dxl022
This page titled 2.3: Instruments of Microscopy is shared under a CC BY 4.0 license and was authored, remixed, and/or curated by OpenStax via source content that was edited to the style and standards of the LibreTexts platform.
52. Sancho D, Reis e Sousa C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol. (2012) 30:491–529. doi: 10.1146/annurev-immunol-031210-101352.Signaling
11. Lehmann CHK, Heger L, Heidkamp GF, Baranska A, Lühr JJ, Hoffmann A, et al. Direct delivery of antigens to dendritic cells via antibodies specific for endocytic receptors as a promising strategy for future therapies. Vaccines. (2016) 4:8. doi: 10.3390/vaccines4020008
Is light on your Ring device too bright or too dim? You can change the Light Settings in the Ring app.
23. Oh J, Shin JS. The role of dendritic cells in central tolerance. Immune Netw. (2015) 15:111–20. doi: 10.4110/in.2015.15.3.111
106. Kel JM, De Geus ED, Van Stipdonk MJ, Drijfhout JW, Koning F, Nagelkerken L. Immunization with mannosylated peptide induces poor T cell effector functions despite enhanced antigen presentation. Int Immunol. (2008) 20:117–27. doi: 10.1093/intimm/dxm123
The use of glycan-based strategies has been substantially tested in pre-clinical and a few clinical settings for the treatment of allergies (Table 1). In these studies, carbohydrate-modified allergens were used to dampen allergic immune responses while installing antigen-specific T cell anergy both in vivo and in vitro (76, 77, 109–112). A notable mention is a study by Sirvent et al where they conjugated non-oxidized mannan from Saccharomyces cerevisae to polymerized grass pollen allergens (PM) and demonstrated that PM-treated human moDCs favor the induction of CD4+CD25highCD127−Foxp3+ Tregs over Th1 cells through PD-L1 signaling, subsequently causing an increase in the IL-10/IL-5 cytokine ratio produced by T cells (76) (Figure 2). PM was captured via the MR and DC-SIGN and proved to be hypoallergenic during in vivo skin prick tests and ex vivo basophil activation tests. The same group demonstrated that this strategy is equally effective in the treatment of canine atopic dermatitis (77). Interestingly, oxidation of mannan impaired the tolerogenic properties of PM shown in both human and mice, emphasizing the importance of the mannan structure for its functional properties (76, 109, 113). Also, Mathiesen et al. used the major birch pollen allergen, Bet v 1, coupled to defined carbohydrate structures and demonstrated that the prophylactic treatment of mice with GalNAc-coupled Bet v 1 significantly reduces IgE responses (78) (Figure 2). This finding suggests that MGL, which recognizes terminal α-and β-linked GalNAc structures, might be involved in the induction of the observed immune responses and may thus qualify as another potential candidate for specific antigen-delivery to DCs for induction of tolerance (114). Cumulatively, available data suggest that targeting DC-SIGN, MR and MGL for specific antigen delivery is a promising strategy to be further explored for the management of dysregulated immune pathologies (Figure 2).
50. Rapoport EM, Khaidukov SV, Gaponov AM, Pazynina GV, Tsygankova SV, Ryzhov IM, et al. Glycan recognition by human blood mononuclear cells with an emphasis on dendritic cells. Glycoconj J. (2018) 35:191–203. doi: 10.1007/s10719-017-9811-6
51. Redelinghuys P, Brown GD. Inhibitory C-type lectin receptors in myeloid cells. Immunol Lett. (2011) 136:1–12. doi: 10.1016/j.imlet.2010.10.005
53. Fehres CM, Unger WWJ, Garcia-Vallejo JJ, van Kooyk Y. Understanding the biology of antigen cross-presentation for the design of vaccines against cancer. Front Immunol. (2014) 5:149. doi: 10.3389/fimmu.2014.00149
25. Proietto AI, Van Dommelen S, Zhou P, Rizzitelli A, D'Amico A, Steptoe RJ, et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci USA. (2008) 105:19869–74. doi: 10.1073/pnas.0810268105
Phase-contrast microscopes use refraction and interference caused by structures in a specimen to create high-contrast, high-resolution images without staining. It is the oldest and simplest type of microscope that creates an image by altering the wavelengths of light rays passing through the specimen. To create altered wavelength paths, an annular stop is used in the condenser. The annular stop produces a hollow cone of light that is focused on the specimen before reaching the objective lens. The objective contains a phase plate containing a phase ring. As a result, light traveling directly from the illuminator passes through the phase ring while light refracted or reflected by the specimen passes through the plate. This causes waves traveling through the ring to be about one-half of a wavelength out of phase with those passing through the plate. Because waves have peaks and troughs, they can add together (if in phase together) or cancel each other out (if out of phase). When the wavelengths are out of phase, wave troughs will cancel out wave peaks, which is called destructive interference. Structures that refract light then appear dark against a bright background of only unrefracted light. More generally, structures that differ in features such as refractive index will differ in levels of darkness (Figure \(\PageIndex{5}\)).
96. Cvetkovic J, Ilic N, Gruden-Movsesijan A, Tomic S, Mitic N, Pinelli E, et al. DC-SIGN signalling induced by Trichinella spiralis products contributes to the tolerogenic signatures of human dendritic cells. Sci Rep. (2020) 10:20283. doi: 10.1038/s41598-020-77497-x
121. Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem. (2008) 283:16693–701. doi: 10.1074/jbc.M709923200
111. Palomares F, Ramos-Soriano J, Gomez F, Mascaraque A, Bogas G, Perkins JR, et al. Pru p 3-glycodendropeptides based on mannoses promote changes in the immunological properties of dendritic and t-cells from LTP-allergic patients. Mol Nutr Food Res. (2019) 63:553. doi: 10.1002/mnfr.201900553
24. Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. (2009) 9:833–44. doi: 10.1038/nri2669




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500