Notice Signs - notice sign
Personal security is one of the most important aspects of business travel and the ironic aspect is that so few business professionals plan for it. While you are away on company travel, you need to make sure that your home and identity are safe while you are away.
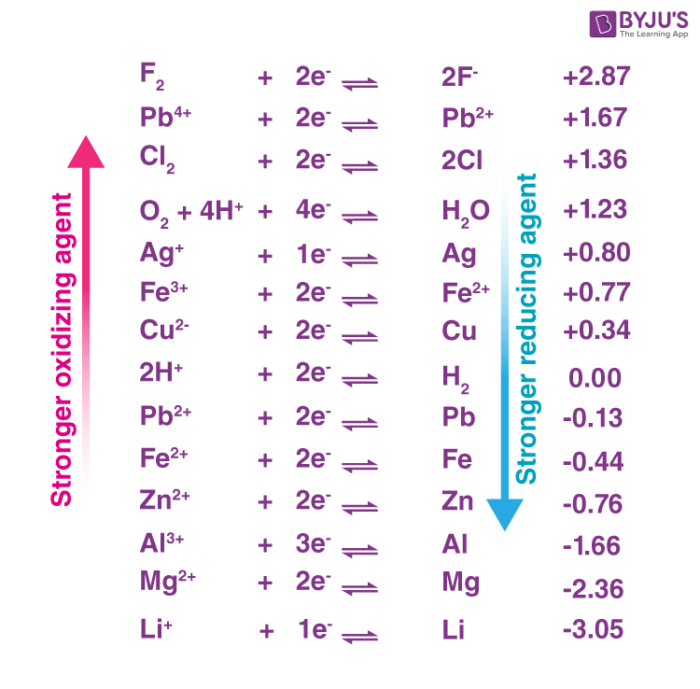
The standard electrode potential of a half-reaction in a redox process provides insight into the oxidizing power of the chemical substance. An illustration ranking some oxidizers in terms of their oxidizing powers is provided below.
An oxidizing agent (often referred to as an oxidizer or an oxidant) is a chemical species that tends to oxidize other substances, i.e. cause an increase in the oxidation state of the substance by making it lose electrons. Common examples of oxidizing agents include halogens (such as chlorine and fluorine), oxygen, and hydrogen peroxide (H2O2).
Hydrogen peroxide is the chemical compound having formula H2O2. It appears to the human eye as a colourless liquid which has a greater viscosity than water. Hydrogen peroxide is the simplest compound having a peroxide functional group with an oxygen-oxygen single bond. It finds its uses as a weak oxidizing agent, disinfectant, and bleaching agent.
Types ofoxidising
A great way to boost your personal security is to have a personal chauffeur when you’re visiting a city. Professional drivers are more trustworthy and reliable than public transportation.
An oxidizing agent is a compound or element that participates in a redox (oxidation-reduction) reaction and accepts electrons from a different species. An oxidant is a chemical compound that easily transfers oxygen or another substance atoms in order to gain an electron.
That lockbox in your hotel room is for more than midnight snacks. Keeping your valuables in a safe place is an absolute necessity when it comes to business travel. No matter how experienced of a traveler you are, out of town visitors are targeted because of their inexperience with the city and the amount of valuables that travelers carry.
Oxidisingexamples
Another important component of getting through airport security is to dress for the airport security line. Wearing shoes that are easily slipped on and off, carrying a bag with a laptop case that can be easily removed, and searching your clothing for metallic items before traveling are great ways to cut down on travel time and increase your personal security. Also, here’s an article by USA Today on airport security trips.
Oxidisingin chemistry
Strongoxidisingagent meaning
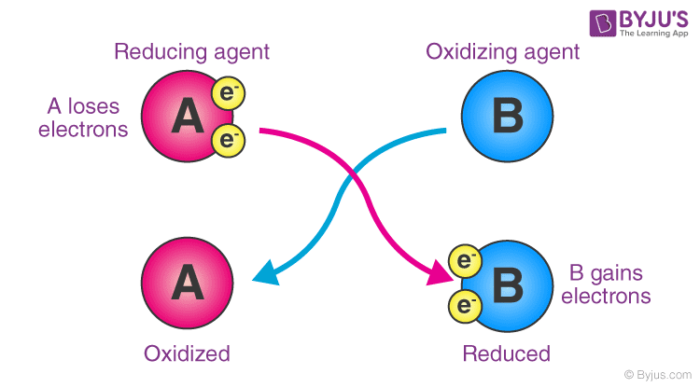
An oxidizing agent is a substance that causes oxidation by accepting electrons and thus becoming reduced. A reducing agent is a substance that causes reduction by losing electrons, causing it to be oxidized.
Oxygen is the element corresponding to the atomic number 8 and is denoted by the symbol ‘O’. It belongs to the chalcogen group of the periodic table and is a highly reactive non- metal with good oxidizing properties. In general, metals tend to form metal oxides by reacting with atmospheric oxygen, due to the strong oxidizing power of oxygen. Oxygen is observed to be a part of a majority of combustion reactions.
You can keep your digital identity safe is by making sure you only do business with companies you know and trust. The digital landscape changes literally daily. By the time this article has been written and posted there will be several new ways that hackers are trying to steal identities. Staying on the cutting edge is difficult, but doing some research before every trip is a good way to stay in touch with modern security.
The statistics show that the most common time for a burglary to happen is between the hours of 6 am and 6 pm. That means that it’s more important to have your house appear to be busy during the day than it is overnight.
When it comes to electron transfers, an oxidizing agent gains electrons while oxidation is the process of losing electrons. Reduction, on the other hand, means gaining electrons. As a result, the oxidizing agent gains electrons from the species being oxidized, implying that it undergoes reduction (as it gains electrons).
Some compounds that exhibit large oxidation states can also be considered good oxidizing agents. Ionic examples include the permanganate ion, the chromate ion, and the dichromate ion. Acidic examples of good oxidizers include nitric acid, perchloric acid, and sulphuric acid. The electronegativity of the molecules increases with the increase in the oxidation states of the atoms, increasing their ability to oxidize other substances.
As an atom-transferring substance – An oxidizing agent is a substance that transfers at least one electronegative atom to a chemical species in a chemical reaction. The transferred atom is typically an oxygen atom. Several combustion reactions and organic redox reactions involve the transfer of an electronegative atom between two reactants.
Oxidisingchemicals
By far the most complicated part of any business transportation is air travel. Airport security is more intense now than ever before. Make sure that you unpack your entire carry-on to check for any restricted items the day before your trip. If you accidentally leave a restricted item in your carry-on and try to get through airport security with it, that can drastically impact your time at the airport.
Oxidisingprocess
Personal alarm systems are a great way to thwart crime. Buying an alarm system is only the first step. The second is making sure that you utilize the signs/stickers your alarm company provides and most importantly—you must utilize the alarm system.
To learn more about oxidizing agents and the part they play in oxidation-reduction reactions, register with BYJU’S and download the mobile application on your smartphone.
A few other examples of elemental oxidizing agents include diatomic oxygen (O2), diatomic chlorine (Cl2), and ozone (O3). These oxidizers are the elemental forms of the second and the third most electronegative elements (oxygen and chlorine respectively), making them good electron acceptors.
Whether or not you have family who live at your house or if you are a single traveler, it is extremely important that your home maintains the look of an occupied household. This means not letting mail accumulate, having lights turned on, and ideally having someone stop by periodically.
It’s also extremely important to lock your luggage when possible. If you are checking bags, it’s important to think about how many different people will handle your luggage by the time that it gets back to you.
As an electron acceptor – They are chemical substances whose atoms remove at least one electron from another atom in a chemical reaction. As per this definition, oxidizing agents are the reactants that undergo reduction in redox reactions. An illustration detailing the electron-accepting properties of oxidizing agents is provided below.
In an article by Shivani Vora at Forbes, they highlight one of the sneakiest ways that hackers can steal your identity, which is by creating dummy wireless hotspots. Once you log in to one, they can access your computer and steal your personal information.
The group 17 elements of the periodic table are collectively referred to as Halogens. They are said to have a strong ability to gain electrons, attributed to their high electronegativities when compared to elements from other groups. This implies that they have the ability to easily attract electrons towards their respective nuclei. Examples of the halogens that are good oxidizing agents include iodine, bromine, chlorine, and fluorine. Fluorine is said to be the strongest elemental oxidizing agent due to its highest electronegativity, as discussed earlier.
Oxidisingsubstance symbol
What isoxidisingagent
In the example illustrated above, the Fe2O3 molecule acts as an oxidizer by transferring an electronegative oxygen atom to the carbon monoxide molecule.
Many organisms make use of electron acceptors, or oxidizers, to collect energy from the redox reactions such as in the process of hydrolysis of glucose.
Oxidizing agents normally exist in their highest possible oxidation states and, therefore, have a strong tendency to gain electrons and undergo reduction. Ions, Atoms, and molecules having a strong affinity towards electrons are considered to be good oxidizers. The stronger the electron affinity, the greater the oxidizing power.
By accepting electrons from other substances, oxidizing agents cause their oxidation states to become more positive. Oxidizing agents are reduced as well.
Elemental fluorine is said to be the strongest elemental oxidizing agent. This is perhaps due to the fact that fluorine is the most electronegative element in the modern periodic table, and therefore exerts the strongest attractive force on electrons amongst all the elements. In fact, the oxidizing power of diatomic fluorine (F2) is strong enough to cause metals such as asbestos and quartz (and even molecules, such as water) to burst into flames when exposed to it.
Many other oxidizing agents are commonly used industrially as well as in the day-to-day lives of humans. Examples include household bleach (NaClO), Potassium Nitrate (KNO3), and Sulphuric acid (H2SO4).
Here, substance ‘A’ undergoes oxidation, resulting in an increase in its oxidation number. On the other hand, the oxidation state of substance ‘B’ becomes smaller (since it gains electrons by undergoing reduction).




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500