All Makes All Models OER - Body Mount Bushings - body mounts
Although most aerosols remain suspended in the atmosphere for short periods—typically between four days and a week—they can travel vast distances. Particles moving with the atmosphere at 5 meters (16.4 feet) per second will travel thousands of kilometers in a week. Dust plumes from the Sahara frequently cross the Atlantic and reach the Caribbean. Winds sweep a mixture of Asian aerosols—particularly dust from the Gobi desert and pollution from China—east over Japan and toward the central Pacific Ocean. Smoke from wildfires in Siberia and Canada can find its way to the Arctic ice cap.
Meanwhile, the eastern portion of the United States and urban areas in Europe are hotspots for the production of human-made aerosols. Plumes of industrial aerosols — typically sulfates from coal power plants and black and organic carbon from vehicle traffic — rise from cities such as New York, Pittsburgh, London, and Berlin.
Dust from North Africa spreads over the Atlantic Ocean on July 1, 2009 (from 00:00 to 18:00 Universal Time) in this series of images derived from a computer model of aerosol movement. Click for animation. (NASA maps by Pete Colarco and Robert Simmon, based on GEOS-5 model data.)
The safety data sheet tells you how to store hazardous substances safely to minimise the risk of harm to individuals, property and the environment.
Automobiles, incinerators, smelters, and power plants are prolific producers of sulfates, nitrates, black carbon, and other particles. Deforestation, overgrazing, drought, and excessive irrigation can alter the land surface, increasing the rate at which dust aerosols enter the atmosphere. Even indoors, cigarettes, cooking stoves, fireplaces, and candles are sources of aerosols.
Different aerosols scatter or absorb sunlight to varying degrees, depending on their physical properties. Climatologists describe these scattering and absorbing properties as the “direct effect” of aerosols on Earth’s radiation field. However, since aerosols comprise such a broad collection of particles with different properties, the overall effect is anything but simple.
Dark aerosols dramatically change the reflectivity of the Earth’s surface when they land on snow. Black ash covered the summit of New Zealand’s Mount Ruapehu after an eruption in 2007, but was soon covered by fresh snow. Long-term accumulation of black carbon aerosols in the Arctic and Himalaya is leading to increased melting of snow. (Photograph ©2007, New Zealand GeoNet.)
Chemical manufacturers, sellers, and distributors are required by law to provide safety data sheets. They’re typically included in the chemical’s packaging but they can also be viewed on the manufacturer’s website. You can download the safety data sheets for every chemical we supply here.
Scientists believe the cooling from sulfates and other reflective aerosols overwhelms the warming effect of black carbon and other absorbing aerosols over the planet. Models estimate that aerosols have had a cooling effect that has counteracted about half of the warming caused by the build-up of greenhouse gases since the 1880s. However, unlike many greenhouse gases, aerosols are not distributed evenly around the planet, so their impacts are most strongly felt on a regional scale.
The Sun provides the energy that drives Earth’s climate, but not all of the energy that reaches the top of the atmosphere finds its way to the surface. That’s because aerosols—and clouds seeded by them—reflect about a quarter of the Sun’s energy back to space.
NASA co-sponsors a global network of ground sensors called the Aerosols Robotic Network, or AERONET, which is comprised of more than 200 carefully calibrated sun photometers measuring aerosol optical depth around the world. The relatively simple instruments deduce the amount and type of aerosol in the sky by measuring the intensity of light under cloud-free conditions. Aircraft and other ground stations deploy in situ instruments that measure certain aerosol properties with greater accuracy, but less frequently.
While anyone can access safety data sheets online, these documents aren’t really intended for the general public. They’re primarily designed to be used by anyone working with chemicals in an occupational setting, although they can also be used for research purposes.
To help minimise the risk and keep everyone safe, safety data sheets must be updated regularly. Manufacturers, large-scale distributors and resellers, importers and employees are all required to record and update any new information in their inventories.
Natural aerosols—often sulfates, sea salt or ammonium salts—are the most common condensation nuclei in pristine environments. Polluted air, in contrast, usually contains much higher concentrations of water-soluble particles, which means pollution-rich clouds tend to have more numerous, but smaller, droplets. The small droplets make polluted clouds look brighter than they would otherwise be. Just as many bits of crushed ice give light more surfaces to reflect off—appearing brighter than a solid cube of ice—if the water in a cloud is divided into a larger number of smaller droplets, it will scatter more light and become more reflective.
Safety data sheets include details about the chemical’s composition as well as the health and environmental hazards it poses. Alongside this, they contain occupational safety instructions and advice on managing spillages. This helps to ensure that anyone handling or using the substance can do so safely. Crucially, an SDS also provides vital information about how to apply first aid if someone inhales, ingests or comes into contact with the chemical.
Pure sulfates and nitrates reflect nearly all radiation they encounter, cooling the atmosphere. Black carbon, in contrast, absorbs radiation readily, warming the atmosphere but also shading the surface. Organic carbon, sometimes called brown carbon or organic matter, has a warming influence on the atmosphere depending on the brightness of the underlying ground. Dust impacts radiation to varying degrees, depending on the composition of the minerals that comprise the dust grains, and whether they are coated with black or brown carbon. Salt particles tend to reflect all the sunlight they encounter.
Overall, clouds are thought to cool Earth’s surface by shading about 60 percent of the planet at any one time and by increasing the reflectivity of the atmosphere. Given that, just a 5 percent increase in cloud reflectivity could compensate for the entire increase in greenhouse gases from the modern industrial era in the global average. Likewise, long-term decreases in cloudiness could have major impacts.
Although it became clear about 40 years ago that aerosols could affect climate, the measurements needed to establish the magnitude of such effects—or even whether specific aerosol types warm or cool the surface—were lacking.
Most elementary school students learn that clouds form when enough water vapor condenses. That’s true, but aerosols play a critical role in the process. In fact, most clouds owe their existence to aerosols that serve as the tiny “seeds,” called cloud condensation nuclei.
This map shows the global distribution of aerosols and the proportion of those aerosols that are large or small. Intense colors indicate a thick layer of aerosols. Yellow areas are predominantly coarse particles, like dust, and red areas are mainly fine aerosols, like smoke or pollution. Gray indicates areas with no data. (NASA map by Robert Simmon, based on MODIS data from NASA Earth Observations.)
Although chemical safety data sheets need to be regularly reviewed and modified, in most cases, no major updates are normally required. This effectively means an SDS can be valid for up to five years or as long as the chemical has not passed the expiration date on the label.
Despite great progress, many questions remain about the competing impacts of aerosols. Measuring particles within clouds remains challenging. Different types of particles can clump together to form hybrids that are difficult to distinguish. And changes in humidity or temperature can cause drastic changes in how certain aerosols behave and interact with cloud droplets.
Take a deep breath. Even if the air looks clear, it’s nearly certain that you’ll inhale tens of millions of solid particles and liquid droplets. These ubiquitous specks of matter are known as aerosols, and they can be found in the air over oceans, deserts, mountains, forests, ice, and every ecosystem in between. They drift in Earth’s atmosphere from the stratosphere to the surface and range in size from a few nanometers—less than the width of the smallest viruses—to several several tens of micrometers—about the diameter of human hair. Despite their small size, they have major impacts on our climate and our health.
Aerosols in pharmacy
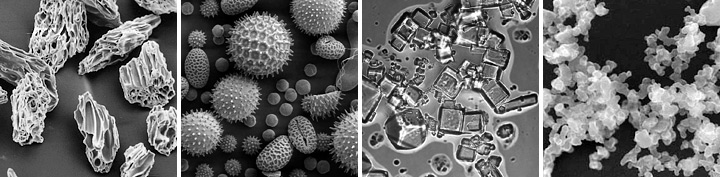
These scanning electron microscope images (not at the same scale) show the wide variety of aerosol shapes. From left to right: volcanic ash, pollen, sea salt, and soot. [Micrographs courtesy USGS, UMBC (Chere Petty), and Arizona State University (Peter Buseck).]
All chemicals that pose a potential health or environmental hazard are required to have safety data sheets. This includes chemicals that are corrosive, toxic, flammable, explosive, and carcinogenic. Both oxidising and pressurised gases also need an accompanying SDS.
Newer instruments also provide insight into properties of light that AVHRR couldn’t detect. The Cloud Aerosol Lidar and Infrared Pathfinder Satellite Observer (CALIPSO) uses a laser-based technology that provides detailed vertical profiles of aerosol plumes and clouds. A French instrument, the Polarization and Directionality of the Earth’s Reflectances (POLDER) offers insight into the orientation—or polarization—of light waves as they move through the atmosphere. This previously unmeasured quantity has proven helpful in retrieving particle type information. An instrument even more sensitive to the polarization of light, the Aerosol Polarimetery Sensor (APS), will gather additional insights after its launch aboard NASA’s Glory mission in 2011.
Over time, aerosol emissions have changed significantly. In Asia, anthropogenic emissions have increased in recent decades as urbanization and industrialization has proceeded at a breakneck pace. By contrast, aerosols have declined in North America and Europe as factories have moved to developing countries and Western nations have adopted more stringent clean air regulations.
Dust storms (top) and wildfires (bottom) are significant, if transient, sources of aerosols. (NASA images by Jeff Schmaltz, MODIS Rapid Response.)
Whereas aerosols can influence climate by scattering light and changing Earth’s reflectivity, they can also alter the climate via clouds. On a global scale, these aerosol “indirect effects” typically work in opposition to greenhouse gases and cause cooling. While greenhouse gases disperse widely and have a fairly consistent impact from region to region, aerosol effects are less consistent, partly because of how the particles affect clouds.

Starting in 2011, the Glory mission will enable scientists to better characterize the types and distribution of aerosols. (NASA image by Ryan Zuber, GSFC Scientific Visualization Studio.)
Climatologists typically use another set of labels that speak to the chemical composition. Key aerosol groups include sulfates, organic carbon, black carbon, nitrates, mineral dust, and sea salt. In practice, many of these terms are imperfect, as aerosols often clump together to form complex mixtures. It’s common, for example, for particles of black carbon from soot or smoke to mix with nitrates and sulfates, or to coat the surfaces of dust, creating hybrid particles.
The effect of aerosols on clouds is clearly visible in ship tracks—the dense, bright marine clouds formed by the exhaust of passing ships. This natural-color image shows the bright white clouds (top) and a map of cloud droplet radius (bottom). Where ship exhaust mixed with the cloud layer, droplets became much smaller. (NASA image by Jesse Allen and Robert Simmon, based on data from MODIS.)
Most importantly, the quantitative uncertainties in the amount of aerosols, and especially aerosol properties, need to be reduced. Only better measurements—and the more sophisticated computer modeling such measurements make possible—will provide the critical information that scientists need to fully integrate aerosol impacts into climate models and to reduce uncertainty about how the climate will change.
Interactions between smoke and clouds are complex. In some cases, smoke and other aerosols help cloud formation. In others, smoke suppresses clouds. Both effects are apparent in this natural-color satellite image of the Brazilian Amazon, acquired on August 31, 2010. (NASA image by Jeff Schmaltz, MODIS Rapid Response.)
Aerosol optical depth is the fundamental measurement of quantity and distribution of aerosols. This map shows the average distribution of aerosols from June 2000 through May 2010, measured by the Multi-angle Imaging Spectroradiometer (MISR). Red indicates high concentrations of aerosols, beige indicates low concentrations. (NASA map by Robert Simmon, based on MISR data.)
Alongside this, you’ll find warnings about other substances that may potentially react with the chemical you’re handling. You can read more about how to handle hazardous chemicals here.
However, the most aerosol-laden air in the United States today pales in comparison to Asia. Satellites can detect a visible pall of aerosol clouds over Bangladesh, northern India, and northern Pakistan—an area called the Indo-Gangetic plain, especially during the pre-Monsoon season. The aerosol layer is comprised of complex mixtures of dust blowing from the Thar Desert and pollution from the densely populated plain. In eastern China, fast-growing cities such as Beijing also produce heavy blankets of aerosol.
Safety data sheets are usually enclosed in the chemical’s packaging. Some manufacturers also include an email attachment in the order confirmation. If you still can’t find the SDS, you can download a copy from the manufacturer’s website. You can find our MSDS here.
Different specialists describe the particles based on shape, size, and chemical composition. Toxicologists refer to aerosols as ultrafine, fine, or coarse matter. Regulatory agencies, as well as meteorologists, typically call them particulate matter—PM2.5 or PM10, depending on their size. In some fields of engineering, they’re called nanoparticles. The media often uses everyday terms that hint at aerosol sources, such as smoke, ash, and soot.
Read on to learn more about exactly what a safety data sheet is, the type of information it contains, and how to access them.
Although most aerosols reflect sunlight, some also absorb it. An aerosol’s effect on light depends primarily on the composition and color of the particles. Broadly speaking, bright-colored or translucent particles tend to reflect radiation in all directions and back towards space. Darker aerosols can absorb significant amounts of light.
Sea salt and dust are two of the most abundant aerosols, as sandstorms whip small pieces of mineral dust from deserts into the atmosphere and wind-driven spray from ocean waves flings sea salt aloft. Both tend to be larger particles than their human-made counterparts.
What are aerosols in the atmosphere
The bulk of aerosols—about 90 percent by mass—have natural origins. Volcanoes, for example, eject huge columns of ash into the air, as well as sulfur dioxide and other gases, yielding sulfates. Forest fires send partially burned organic carbon aloft. Certain plants produce gases that react with other substances in the air to yield aerosols, such as the “smoke” in the Great Smoky Mountains of the United States. Likewise in the ocean, some types of microalgae produce a sulfurous gas called dimethylsulfide that can be converted into sulfates in the atmosphere.
Many chemicals are hazardous in some way, for example, they might be corrosive, toxic, explosive, or carcinogenic. It’s therefore essential to read and understand the SDS before working with any kind of hazardous chemical. This will help to reduce the risk of an accident, injury or environmental damage.
A safety data sheet, or SDS, is a technical document that contains specific information about a particular chemical, substance, compound, mixture, or product in a container. It’s also known as a material safety data sheet (MSDS) or product safety data sheet (PSDS).
Aerosol examples
This impact of aerosols is clearly visible in ship tracks, bright streaks in marine clouds that look like airplane contrails. In the absence of ships, sea salt particles and the natural sulfates produced by phytoplankton seed most marine clouds. However, the exhaust from ship smokestacks make trails of sulfates and other aerosols that form long, bright clouds.
The United Nations has developed a standardisation system for identifying chemical hazards. The classifications are included in the Globally Harmonised System, which helps to avoid confusion among international manufacturers and traders.
Types of aerosols
Depending on the season and weather conditions, surges of aerosols can make their way into the atmosphere almost anywhere on Earth. In the Northern Hemisphere, plumes of mineral dust swirl over deserts and arid regions. In the Southern Hemisphere, slash-and-burn agriculture in the Amazon and Central Africa releases large amounts of smoke and soot. Fires, some sparked by lightning and some by human activity, leave large patches of forest ablaze during summers in Canada, Russia, and the United States.
The safe handling instructions include information on how to properly transport and use the chemical safely. They also contain guidance on maintaining the right ambient temperature and other environmental factors.
Aerosols also have complex effects on clouds and precipitation. Broadly speaking, aerosols are thought to suppress precipitation because the particles decrease the size of water droplets in clouds. However, under some environmental conditions, aerosols can lead to taller clouds that are more likely to produce lightning and strong downpours. In a few places, meteorologists have even detected a cycle in which the frequency of thunderstorms is connected to mid-week peaks in aerosol emissions.
Emergency measures include first aid, firefighting, containment, and clean-up instructions. Depending on the type of chemical involved, it may be necessary to neutralise a spillage or add other chemicals to render it non-harmful.
Aerosols play an important role in Earth’s climate. Most aerosols are brighter than land or ocean, and cool the Earth by reflecting sunlight back to space. (NASA astronaut photograph ISS024-E-15122.)
Older safety data sheet formats vary depending on the source and statutory requirements. However, the new internationally standardised SDS format, as documented in the ANSI Standard Z400, is arranged into 16 key sections.
Chemical safety data sheets are technical documents that provide comprehensive information about the composition of manufactured chemicals and the hazards they pose. An SDS includes occupational instructions to help ensure chemicals are handled, stored and used correctly. They also contain details of emergency measures relating to first aid, spillages and firefighting. Always make sure you’ve read and understood the relevant safety data sheet before working with any kind of hazardous chemical.
Satellite instruments such as CALIPSO can measure the distribution of aerosols in a vertical slice of the atmosphere. This image shows a dust plume off the west coast of Africa on April 22, 2010. (NASA image by Jesse Allen and Robert Simmon, based on data from MODIS and CALIPSO.)
Brighter clouds, in turn, block sunlight from reaching Earth’s surface, shading the planet and producing net cooling. This cloud brightening effect—called the “cloud albedo effect”—may have a big impact on the climate, though only in recent years has it been possible to start quantifying the effect.
Aerosol medication
Some chemicals such as corrosive acids and bases need to be stored in inert containers like plastic and glass bottles. The temperature, location, and type of cabinet must also be compatible with the chemicals.
The situation has improved considerably. Today scientists use an array of satellite, aircraft, and ground-based instruments to monitor aerosols. Radiometers—instruments that quantify the amount of electromagnetic radiation (light)—are among the most important tools available. The key quantity they report is aerosol optical depth (AOD), a measure of the amount of light that aerosols scatter and absorb in the atmosphere (and generally prevent from reaching the surface). An optical depth of less than 0.05 indicates a clear sky with relatively few aerosols and maximum visibility, whereas a value of 1 indicates hazy conditions. Optical depths above 2 or 3 represent very high concentrations of aerosols.
Land-based and airborne instruments complement NASA’s satellites. AERONET is a global network of sun photometers that measures aerosols from the ground, such as this station in the Canadian Arctic (top). The Global Hawk Uninhabited Aerial Vehicle (bottom) can carry several instruments to measure aerosols from the air. Photograph by Ovidiu Pancrati, ©2007 CANDAC. Lower photograph by Tony Landis/NASA.)
Large volcanic eruptions may lift sulfate aerosols into the stratosphere, which usually cools the global climate for the following year or two. (Graph by Robert Simmon, based on aerosol data from GISS and temperature data from the UAE CRU.)
Aerosol vape
The first satellite instrument capable of crudely monitoring aerosol optical depth from space—the Advanced Very High Resolution Radiometer (AVHRR)—retrieved optical depth from measurements in the visible and near-infrared spectrum, beginning in the late 1970s. AVHRR, a passive radiometer, measured the intensity of sunlight as it reflected off aerosols, using the dark ocean as the background.
The purpose of a safety data sheet (SDS) is to provide vital information on how to store, handle, use, and dispose of chemicals correctly. It can help anyone working with hazardous substances to understand the specific risks posed by certain chemicals.
Satellites provide a critical global perspective for understanding how aerosols affect Earth’s climate, but they are far from the only source of information. Networks of ground-based sensors are used to validate satellite measurements and offer some of the most accurate measurements of AOD available.
Safety data sheets aren’t really designed for use by the general public. Rather, they usually focus on the hazards posed by chemicals in an occupational setting such as a laboratory or a factory.
What Is aerosol in Chemistry
Aside from descriptions, pictograms are also standardised. Chemical hazards can be classified into several categories such as corrosive, flammable, explosive, toxic, and carcinogenic. See the diagram below.
Nature generates broad swaths of particles—detectable by satellites—over both water and land. The strong winds of the “roaring forties” latitudes, for example, create a heavy band of airborne salt north of Antarctica. A thinner and more evenly dispersed veil of aerosols—primarily salt from whitecaps and sulfates from microalgae—usually covers most of the world’s oceans. Over land, massive plumes of dust blow above deserts.
The details of aerosol indirect effects are only partially understood, as most instruments cannot measure aerosols within clouds. Climatologists consider the role of clouds to be the largest single uncertainty in climate prediction. Less than a third of the models participating in the Fourth Intergovernmental Panel on Climate Change (IPCC) included indirect aerosol effects, even in a very limited way, and those considered only sulfate aerosols.
The blog on chemicals.co.uk and everything published on it is provided as an information resource only. The blog, its authors and affiliates accept no responsibility for any accident, injury or damage caused in part or directly from following the information provided on this website. We do not recommend using any chemical without first consulting the Material Safety Data Sheet which can be obtained from the manufacturer and following the safety advice and precautions on the product label. If you are in any doubt about health and safety issues please consult the Health & Safety Executive (HSE).
Chemicals are identified based on their chemical composition and product identifier. The product identifier also stipulates what the chemical is used for. For example, crude oil is used for refining purposes, which means it requires further processing.
A safety data sheet provides comprehensive information on the chemical composition, health and environmental hazards, safety instructions for handling and storage, and first aid and emergency protocols.
The SDS and labels must be updated every six months or whenever new information about the chemical is published in scientific papers or by the regulatory authorities.
The western portion of the United States is comparatively clear, though some areas experience aerosol loads that rival the worst conditions in the East. Industrial aerosols, dust, and wildfire smoke frequently pollute the air in the Los Angeles Basin. Agriculture can produce heavy loads of soil dust, especially in California’s San Joaquin and Imperial valleys, and the largest localized source of dust in the western U.S. is Owens Dry Lake, a river bed that was drained to provide water for LA. Likewise, the port of Houston has some of the most aerosol-laden air in the world.
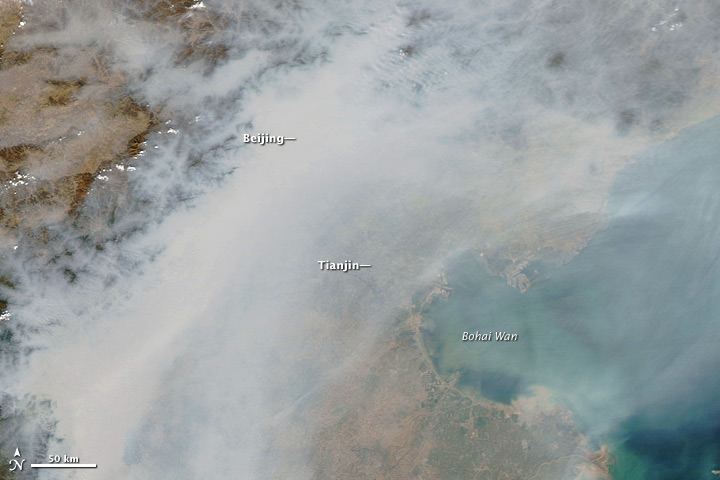
Beijing was completely obscured by air pollution on October 9, 2010. This natural-color image was acquired by MODIS aboard the Aqua satellite. (NASA image by Jeff Schmaltz, MODIS Rapid Response.)
Aerosol monitoring instruments also measure single scattering albedo (SSA), the fraction of light that is scattered compared to the total. Values for most aerosols range from about 0.7 for very absorbing particles to 1 for aerosols that only scatter light.
Aerosols—tiny, airborne solid & liquid particles—are present throughout the atmosphere and largely responsible for hazy skies, as in this photograph of Shenzen, China. (Photograph ©2010 pseudo-san.)
Clouds in clean air are composed of a relatively small number of large droplets (left). As a consequence, the clouds are somewhat dark and translucent. In air with high concentrations of aerosols, water can easily condense on the particles, creating a large number of small droplets (right). These clouds are dense, very reflective, and bright white. This influence of aerosols on clouds is called the “indirect effect,” and is a large source of uncertainty in projections of climate change. (NASA image by Robert Simmon.)
Despite considerable advances in recent decades, estimating the direct climate impacts of aerosols remains an immature science. Of the 25 climate models considered by the Fourth Intergovernmental Panel on Climate Change (IPCC), only a handful considered the direct effects of aerosol types other than sulfates.
Aerosols can have a major impact on climate when they scatter light. In 1991, the eruption of Mount Pinatubo in the Philippines ejected more than 20 million tons of sulfur dioxide—a gas that reacts with other substances to produce sulfate aerosol—as high as 60 kilometers (37 miles) above the surface, creating particles in the stratosphere. Those bright particles remained above the clouds and didn’t get washed from the sky by rain; they settled only after several years.
Aerosol type plays an important role in determining how aerosols affect clouds. Whereas reflective aerosols tend to brighten clouds and make them last longer, the black carbon from soot can have the opposite effect. Studies of pollution over the Indian Ocean and biomass burning smoke in the Amazon have shown that the black carbon warms the surrounding atmosphere and can cause cloud droplets to evaporate. This process, called the “semi-direct effect,” turns clouds into a smoky haze that suppresses precipitation.
In addition to scattering or absorbing radiation, aerosols can alter the reflectivity, or albedo, of the planet. Bright surfaces reflect radiation and cool the climate, whereas darker surfaces absorb radiation and produce a warming effect. White sheets of sea ice, for example, reflect a great deal of radiation, whereas darker surfaces, such as the ocean, tend to absorb solar radiation and have a net warming effect.
Aerosol Spray
The remaining 10 percent of aerosols are considered anthropogenic, or human-made, and they come from a variety of sources. Though less abundant than natural forms, anthropogenic aerosols can dominate the air downwind of urban and industrial areas.
In addition to the safety data sheet, hazardous products must have corresponding symbols that immediately show what type of hazard they pose. As these symbols are standardised, anyone who is properly oriented with them will instantly be able to identify the potential hazard.
Aerosols, particularly black carbon, can alter reflectivity by depositing a layer of dark residue on ice and other bright surfaces. In the Arctic especially, aerosols from wildfires and industrial pollution are likely hastening the melting of ice.
Black carbon aerosols, similar to the soot in a chimney, absorb sunlight rather than reflecting it. This warms the layer of the atmosphere carrying the black carbon, but also shades and cools the surface below. (Maps adapted from Chung, 2005.)
Over subsequent decades, the instruments have grown more sophisticated and made it possible to study aerosols over the land as well. Newer radiometers such as the Multi-angle Imaging Spectroadiometer (MISR) and the Moderate Resolution Imaging Spectroradiomer (MODIS) are capable of viewing aerosols at many more angles and wavelengths, providing more accurate results.
Desert dust, volatile organic compounds from vegetation, smoke from forest fires, and volcanic ash are natural sources of aerosols. (Photographs copyright (left to right) Western Sahara Project, Jonathan Jessup, Vox, and Ludie Cochrane.)
Fossil fuel combustion produces large amounts of sulfur dioxide, which reacts with water vapor and other gases in the atmosphere to create sulfate aerosols. Biomass burning, a common method of clearing land and consuming farm waste, yields smoke that’s comprised mainly of organic carbon and black carbon.
However, aerosols are distributed around the planet differently than greenhouse gases, so the effects do not simply cancel each other. Parsing out how clouds—as well as feedback cycles involving clouds—affect regional climate systems remains a high priority for climatologists.
Your message is sent directly to our dedicated customer service support team where it is distributed to the department best suited to help you. During office hours one of our staff members will promptly email you back or give you a quick call to discuss your requirements or respond to your question. All without obligation or sales pressure. We are here to help you, and are dedicated to “above and beyond” levels of customer service.
Climatologists predicted global temperatures would drop as a result of that global sulfate infusion. They were right: Following the eruption, global temperatures abruptly dipped by about a half-degree (0.6°C) for about two years. And Pinatubo isn’t a unique event. Large, temperature-altering eruptions occur about once per decade.
Current estimates suggest the cooling driven by aerosol indirect effects is less than half as much as the warming caused by greenhouse gases when averaged over the globe. But these indirect effects are highly uncertain and vary considerably in space and time. Therefore, on smaller space and time scales, the climate effects of aerosols can be significant.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500