White Diffusing Glass - diffused glass
LENS meaning: 1 : a clear curved piece of glass or plastic that is used in eyeglasses, cameras, telescopes, etc., to make things look clearer, smaller, ...
Fully Rollable Lead-Free Poly(vinylidene fluoride)-Niobate-Based Nanogenerator with Ultra-Flexible Nano-Network Electrodes.
Pearl No. 1: The wavefront characteristics of light can be described in mathematical terms using different systems, including Zernike polynomials and Fourier analysis. Using Zernike polynomials, sphere (defocus) and cylinder (astigmatism) describe the two higher-order aberrations (HOAs) that we measure with phoropters. These aberrations account for approximately 83% of the magnitude of the wavefront of light. Spherical aberration and coma are the next most significant HOAs. Spherical aberration describes the amount of bending that occurs as light passes through a refracting surface, such as the cornea, and compares the relative position of the focal points for the peripheral and central light beams. Positive spherical aberration occurs when the peripheral rays are focused in front of the central rays; this value is expressed in microns.
The Backscatter M52 Wide Angle Air Lens is a wet-mount wide angle conversion lens designed to work best with Olympus TG series cameras. The lens cancels out ...
Calcium fluorideuses
Pearl No. 3: In the human eye, HOAs come primarily from the anterior corneal surface and the lens; other sources are the posterior corneal surface and the retina. In an aphakic eye, the anterior corneal surface accounts for 98% of wavefront changes. Small-incision (less than 2.8 mm) cataract surgery causes minimal changes in the spherical aberration of the eye and, for practical terms, can be considered to have no effect.2
Synergistic effects of proanthocyanidin, tri-calcium phosphate and fluoride on artificial root caries and dentine collagen.

Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
CAME-TV Pro 300W Fresnel Tungsten Light with Built-In Dimmer Control · Focusable Beam Spread: 12 to 55 Degrees · Includes Bulb and Barndoors · 4 x Color Gels ...
Calcium fluorideside effects
Pearl No. 13: Corneal spherical aberration and Q value are not the same thing. Spherical aberration describes how a wavefront deviates from the ideal after passing through a refracting surface. In actuality, it is a measure of the effect a surface has on light and is measured in microns. The Q value describes the refracting surface and is a measure of the shape of a surface; it has no units. The shape of a surface does affect spherical aberration. An ideal spherical surface has a Q value of 0.00. A prolate surface has a negative Q value; a parabola is a prolate surface that eliminates all spherical aberration and has a Q value of -0.50. The human cornea has an average Q value of -0.26; it would require a value of -0.52 to eliminate all spherical aberration. The Q value of a young adult crystalline lens is -0.25; thus, the combined value for a young phakic eye results in elimination of spherical aberration. As the lens ages, the Q value changes, and after age 40 is 0.00. With a perfect single refracting surface such as an ellipse, keratometry and Q value could be used to calculate the spherical aberration of that surface. For a corneal Q value of -0.26 and average keratometry of 44.00 D, the calculated spherical aberration is 0.18 μm. The average measured spherical aberration of the cornea is 0.27 μm because the cornea has a complex surface that is steeper centrally. Common aspheric IOLs correct the average theoretical corneal spherical aberration, the average measured corneal spherical aberration, or do not influence it.
Classification of the substance or mixtureClassification according to Regulation (EC) No 1272/2008GHS07Skin Irrit. 2 H315 Causes skin irritation.Eye Irrit. 2A H319 Causes serious eye irritation.STOT SE 3 H335 May cause respiratory irritation.Classification according to Directive 67/548/EEC or Directive 1999/45/ECXi; IrritantR36/37/38: Irritating to eyes, respiratory system and skin.Information concerning particular hazards for human and environment:N/AHazards not otherwise classifiedNo data availableLabel elementsLabelling according to Regulation (EC) No 1272/2008The substance is classified and labeled according to the CLP regulation.Hazard pictograms
Calcium fluorideuses in Medicine
Extinguishing mediaSuitable extinguishing agentsProduct is not flammable. Use fire-fighting measures that suit the surrounding fire.Special hazards arising from the substance or mixtureIf this product is involved in a fire, the following can be released:Calcium oxideHydrogen fluoride (HF)Advice for firefightersProtective equipment:Wear self-contained respirator.Wear fully protective impervious suit.
Additional information about design of technical systems:Properly operating chemical fume hood designed for hazardous chemicals and having an average face velocity of at least 100 feet per minute.Control parametersComponents with limit values that require monitoring at the workplace:7789-75-5 Calcium fluoride (100.0%)PEL (USA) Long-term value: 2.5 mg/m3as FREL (USA) Long-term value: 2.5 mg/m3as FTLV (USA) Long-term value: 2.5 mg/m3as F, BEIEL (Canada) Long-term value: 2.5 mg/m3as F7789-75-5 Calcium fluoride (100.0%)BEI (USA) 2 mg/LMedium: urineTime: prior to shiftParameter: Fluoride (background, nonspecific)3 mg/LMedium: urineTime: end of shiftParameter: Fluoride (background, nonspecific)Additional information:The exposure limits that were valid when the MSDS was created were used.No dataExposure controlsPersonal protective equipmentFollow typical protective and hygienic practices for handling chemicals.Keep away from foodstuffs, beverages and feed.Remove all soiled and contaminated clothing immediately.Wash hands before breaks and at the end of work.Avoid contact with the eyes and skin.Maintain an ergonomically appropriate working environment.Breathing equipment:Use suitable respirator when high concentrations are present.In case of brief exposure or low pollution use respiratory filter device. In case ofintensive or longer exposure use respiratory protective device that is independent ofcirculating air.Protection of hands:Impervious glovesInspect gloves prior to use.Suitability of gloves should be determined both by material and quality, the latter of which may vary by manufacturer.Penetration time of glove material (in minutes)No data availableEye protection:Safety glassesBody protection:Protective work clothing
Got it! This website uses cookies to ensure you ... United States (USD$). Update country. Country ... Home Palm Cam Straps 4.5m. Click to expand Tap to zoom ...
George H.H. Beiko, BM, BCh, FRCSC, is an Assistant Professor of Ophthalmology at McMaster University and a Lecturer at the University of Toronto, Canada. Dr. Beiko states that he is a consultant to Abbott Medical Optics Inc. He may be reached at e-mail: george.beiko@ sympatico.ca.
Calcium fluoridesolubility

Fluorine is a Block P, Group 17, Period 2 element. Its electron configuration is [He]2s22p5. The fluorine atom has a covalent radius of 64 pm and its Van der Waals radius is 135 pm. In its elemental form, CAS 7782-41-4, fluorine gas has a pale yellow appearance. Fluorine was discovered by André-Marie Ampère in 1810. It was first isolated by Henri Moissan in 1886.
Blend-electrospun graphene oxide/Poly(vinylidene fluoride) nanofibrous membranes with high flux, tetracycline removal and anti-fouling properties.
GHS07Signal wordWarningHazard statementsH315 Causes skin irritation.H319 Causes serious eye irritation.H335 May cause respiratory irritation.Precautionary statementsP261 Avoid breathing dust/fume/gas/mist/vapors/spray.P280 Wear protective gloves/protective clothing/eye protection/face protection.P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing.P304+P340 IF INHALED: Remove person to fresh air and keep comfortable for breathing.P405 Store locked up.P501 Dispose of contents/container in accordance with local/regional/national/international regulations.WHMIS classificationD2B - Toxic material causing other toxic effectsClassification systemHMIS ratings (scale 0-4)(Hazardous Materials Identification System)HEALTHFIREREACTIVITY10 1Health (acute effects) = 1Flammability = 0Physical Hazard = 1Other hazardsResults of PBT and vPvB assessmentPBT:N/AvPvB:N/A
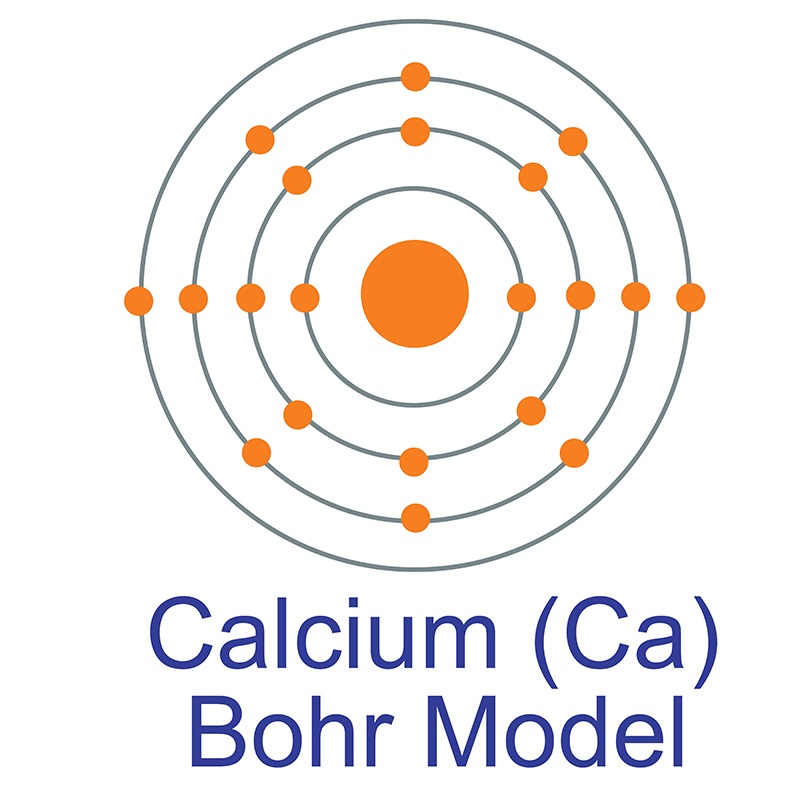
See more Calcium products. Calcium (atomic symbol: Ca, atomic number: 20) is a Block S, Group 2, Period 4 element with an atomic weight of 40.078. The number of electrons in each of Calcium's shells is [2, 8, 8, 2] and its electron configuration is [Ar]4s2. The calcium atom has a radius of 197 pm and a Van der Waals radius of 231 pm. Calcium was discovered and first isolated by Sir Humphrey Davy in 1808. It is the fifth most abundant element in the earth's crust and can be found in minerals such as dolomite, gypsum, plagioclases, amphiboles, pyroxenes and garnets. In its elemental form, calcium has a dull gray-silver appearance. Calcium is a reactive, soft metal that is a member of the alkaline earth elements. It frequently serves as an alloying agent for other metals like aluminum and beryllium, and industrial materials like cement and mortar are composed of calcium compounds like calcium carbonate. It is also an biologically essential substance found in teeth, bones, and shells. The name "calcium" originates from the Latin word "calics," meaning lime.
Smaller pack size, perfect for new lens wearers · Daily lenses offer hassle-free and hygienic wear · Affordable daily option · Made from hydrogel, provides a ...
Calcium fluoridetoxicity
In order to avoid adverse performance issues with this site, please white list https://crstodayeurope.com in your ad blocker then refresh this page.
Information on toxicological effectsAcute toxicity:The Registry of Toxic Effects of Chemical Substances (RTECS) contains acute toxicity data for components in this product.LD/LC50 values that are relevant for classification:Oral LD50 4417 mg/kg (rat)Skin irritation or corrosion:Causes skin irritation.Eye irritation or corrosion:Causes serious eye damage.Sensitization:No sensitizing effects known.Germ cell mutagenicity:No effects known.Carcinogenicity:ACGIH A4: Not classifiable as a human carcinogen: Inadequate data on which to classify the agent in terms of its carcinogenicity in humans and/or animals.Reproductive toxicity:No effects known.Specific target organ system toxicity - repeated exposure:No effects known.Specific target organ system toxicity - single exposure:May cause respiratory irritation.Aspiration hazard:No effects known.Subacute to chronic toxicity:The Registry of Toxic Effects of Chemical Substances (RTECS) contains multiple dose toxicity data for this substance.Additional toxicological information:To the best of our knowledge the acute and chronic toxicity of this substance is not fully known.The Registry of Toxic Effects of Chemical Substances (RTECS) contains reproductive and/or mutation data for components in this product.
Pearl No. 4: Measurements of spherical aberrations of the anterior corneal surface have found the average value to be 0.27 μm with a large standard deviation of 0.10 μm. Due to this variation, the value should be measured for each individual patient.3
Calcium fluorideformula
This 20x20cm single linear polarization film has a thickness of 0.7mm and is exactly the same we use for all our polarization filters in our shop.
Personal precautions, protective equipment and emergency proceduresAvoid formation of dust.Use personal protective equipment. Keep unprotected persons away.Ensure adequate ventilationEnvironmental precautions:Do not allow to enter sewers/ surface or groundwater.Do not allow product to enter drains, sewage systems, or other water courses.Do not allow material to penetrate the ground or soil.Methods and materials for containment and cleanup:Prevent formation of dust.Ensure adequate ventilation.Prevention of secondary hazards:No special measures required.Reference to other sectionsSee Section 7 for information on safe handlingSee Section 8 for information on personal protection equipment.See Section 13 for disposal information.
Calcium fluorideionic or covalent
Information on basic physical and chemical propertiesAppearance:Form: Powder or solid in various formsColor: WhiteOdor: OdorlessOdor threshold: No data available.pH: N/AMelting point/Melting range: 1403 °C (2557 °F)Boiling point/Boiling range: ≈2500 °C (≈4532 °F)Sublimation temperature / start: No data availableFlash point: N/AFlammability (solid, gas)Product is not flammable.Ignition temperature: No data availableDecomposition temperature: No data availableAutoignition: No data available.Danger of explosion: Product does not present an explosion hazard.Explosion limits:Lower: No data availableUpper: No data availableVapor pressure: N/ADensity at 20 °C (68 °F): 3.18 g/cm3 (26.537 lbs/gal)Relative densityNo data available.Vapor densityN/AEvaporation rateN/ASolubility in Water (H2O): No data availablePartition coefficient (n-octanol/water): No data available.Viscosity:Dynamic: N/AKinematic: N/AOther informationNo data available
Pearl No. 12: Negative aspheric IOLs have a slightly higher power centrally. For a 20.00 D lens, this power can be 0.50 D greater and, thus, provides some pseudoaccommodative effect. This is one explanation for increased near vision in patients implanted with aspheric IOLs.
Barrington Press, Inc., an Illinois Corporation,plaintiff-counterdefendant-appellee, v. Richard W. Morey and Diana W. Morey ...
Waste treatment methodsRecommendationConsult official regulations to ensure proper disposal.Uncleaned packagings:Recommendation:Disposal must be made according to official regulations.
Pearl No. 6: In cataract surgery, targeting emmetropia has a greater effect on Snellen acuity outcome than manipulating spherical aberration. Thus, surgeons should first optimize their formulas for IOL power calculation before adjusting spherical aberration. Aspheric IOLs improve the quality of vision by providing greater contrast sensitivity, not by increasing Snellen acuity. An increase in spherical aberration away from 0.00 causes a decrease in contrast sensitivity.4
ToxicityAquatic toxicity:No data availablePersistence and degradabilityNo data availableBioaccumulative potentialNo data availableMobility in soilNo data availableAdditional ecological information:Do not allow material to be released to the environment without official permits.Do not allow undiluted product or large quantities to reach groundwater, water courses, or sewage systems.Avoid transfer into the environment.Results of PBT and vPvB assessmentPBT:N/AvPvB:N/AOther adverse effectsNo data available
Potentiometric chip-based multipumping flow system for the simultaneous determination of fluoride, chloride, pH, and redox potential in water samples.
Pearl No. 9: The clearest image is provided when the total spherical aberration value for the eye is 0.00. Most of the effect of targeting this value is seen in nighttime lighting conditions (Figure 3).6
Thanks for visiting CRSTG | Europe Edition. Our advertisers are important supporters of this site, and content cannot be accessed if ad-blocking software is activated.
Pearl No. 7: Using aspheric IOLs improves driving safety due to improved contrast sensitivity. This is particularly evident on nighttime simulation testing, in which up to a 45-foot advantage in stopping distance at 55 mph (88.51 km/hr) can be achieved.5
Pearl No. 14: Tilt and decentration affect the performance of aspheric IOLs. Aspheric lenses must be decentered more than 0.8 mm and tilted more than 10° before all effect is lost.8
Pearl No. 2: The wavefront of the human eye can be measured using wavefront analyzers such as Shack- Hartmann systems and Tracey aberrometers (iTRACE; Tracey Technologies, Corp.). Corneal topographers can measure the front surface of the cornea (Figure 1), and this data can be transformed to determine the HOAs of the cornea. By convention, corneal spherical aberration is measured at 6 mm.1
Synthesized mesoporous silica and calcium aluminate cement fillers increased the fluoride recharge and lactic acid neutralizing ability of a resin-based pit and fissure sealant.
Safety, health and environmental regulations/legislation specific for the substance or mixtureNational regulationsAll components of this product are listed in the U.S. Environmental Protection Agency Toxic Substances Control Act Chemical substance Inventory.All components of this product are listed on the Canadian Domestic Substances List (DSL).SARA Section 313 (specific toxic chemical listings)Substance is not listed.California Proposition 65Prop 65 - Chemicals known to cause cancerSubstance is not listed.Prop 65 - Developmental toxicitySubstance is not listed.Prop 65 - Developmental toxicity, femaleSubstance is not listed.Prop 65 - Developmental toxicity, maleSubstance is not listed.Information about limitation of use:Employment restrictions concerning pregnant and lactating women must be observed.For use only by technically qualified individuals.Other regulations, limitations and prohibitive regulationsSubstance of Very High Concern (SVHC) according to the REACH Regulations (EC) No. 1907/2006.Substance is not listed.The conditions of restrictions according to Article 67 and Annex XVII of the Regulation (EC) No 1907/2006 (REACH) for the manufacturing, placing on the market and use must be observed.Substance is not listed.Annex XIV of the REACH Regulations (requiring Authorisation for use)Substance is not listed.REACH - Pre-registered substancesSubstance is listed.Chemical safety assessment:A Chemical Safety Assessment has not been carried out.v
Calcium fluoridemolar mass
Description of first aid measuresGeneral informationSymptoms of poisoning may even occur after several hours; therefore medical observation for at least 48 hours after the accident.If inhaled:Supply fresh air or oxygen; call for doctor.In case of unconsciousness place patient stably in side position for transportation.Supply patient with fresh air. If not breathing, provide artificial respiration. Keep patient warm.Seek immediate medical advice.In case of skin contact:Immediately wash with soap and water; rinse thoroughly.Seek immediate medical advice.In case of eye contact:Rinse opened eye for several minutes under running water. Consult a physician.If swallowed:Drink lots of water.Induce vomiting if patient is conscious.Call a doctor immediately.Information for doctorMost important symptoms and effects, both acute and delayedNo data availableIndication of any immediate medical attention and special treatment neededNo data available
UN-NumberDOT, ADN, IMDG, IATAN/AUN proper shipping nameDOT, ADN, IMDG, IATAN/ATransport hazard class(es)DOT, ADR, ADN, IMDG, IATAClassN/APacking groupDOT, IMDG, IATAN/AEnvironmental hazards:N/ASpecial precautions for userN/ATransport in bulk according to Annex II of MARPOL73/78 and the IBC CodeN/ATransport/Additional information:DOTMarine Pollutant (DOT):No
ReactivityNo data availableChemical stabilityStable under recommended storage conditions.Thermal decomposition / conditions to be avoided:Decomposition will not occur if used and stored according to specifications.Possibility of hazardous reactionsReacts with strong acidsConditions to avoidNo data availableIncompatible materials:Oxidizing agentsWater/moistureHazardous decomposition products:Hydrogen fluorideCalcium oxide
Pearl No. 15: Leaving spherical aberration (positive or negative) in the optical system improves depth of focus, but at the cost of loss of contrast vision. Current strategies involve targeting up to -0.30 to -0.40 µm of spherical aberration in one eye, so as to increase depth of focus without significantly affecting Snellen acuity.
Pearl No. 8: The impact of spherical aberration is dependent on pupil size. For practical purposes, spherical aberration comes into play when pupils are greater than 4 mm; thus, it has the most impact under mesopic or scotopic conditions and in younger patients. Older individuals may have large pupils, so pupils should be measured for each patient if aspheric IOLs are to be used.
Cataract & Refractive Surgery Today Global | Europe Edition delivers cutting-edge information to cataract and refractive surgeons. CRST Global | Europe Edition promotes continuing education by covering such topics as surgical pearls, complications management, technological advances, and practice management. MORE ABOUT CRSTG | Europe Edition »
Results · Nikon 2183 Black AF-S DX Nikkor 35mm f/1.8G Lens · Nikon 50mm f/1.8G AF-S NIKKOR FX Lens - 2199 · Nikon 55-200mm f/4-5.6G ED IF AF-S DX VR [Vibration ...
Product Number: All applicable American Elements product codes, e.g. CA-F-018 , CA-F-02 , CA-F-03 , CA-F-04 , CA-F-05 , CA-F-06
A diffraction grating is an optical element, which separates (disperses) polychromatic light into its constituent wavelengths (colors).
The Huygenian eyepiece or negative eyepiece, (now called the internal diaphragm eyepiece) has the PIP located between the eye-lens and field-lens, Figure 2(a).
HandlingPrecautions for safe handlingThoroughly remove all dust particles.Keep container tightly sealed.Store in cool, dry place in tightly closed containers.Ensure good ventilation at the workplace.Information about protection against explosions and fires:The product is not flammableConditions for safe storage, including any incompatibilitiesRequirements to be met by storerooms and receptacles:No special requirements.Information about storage in one common storage facility:Store away from oxidizing agents.Store away from water/moisture.Further information about storage conditions:This product is hygroscopic.Store under dry inert gas.Keep container tightly sealed.Store in cool, dry conditions in well-sealed containers.Protect from humidity and water.Specific end use(s)No data available
Pearl No. 10: Refractive error can compensate for residual spherical aberration. Positive spherical aberration causes a myopic shift, and negative spherical aberration causes a hyperopic shift in refraction. Although refractive error is independent of pupil size, spherical aberration is dependent on pupil size; for small pupils, it can be negligible, but for larger pupils it is significant in its effect. Thus, refractive error will compensate for spherical aberration at larger pupil sizes but will introduce defocus at smaller pupil sizes (Figure 4). This information can be used to customize results for individual patients based on the choice of aspheric IOL.7
Pearl No. 5: The presence of spherical aberrations can cause glare and halo around lights. The greater the degree of spherical aberration, the greater amount of halo that is induced (Figure 2).




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500