What is Mass Spectrometry? - spectrometer
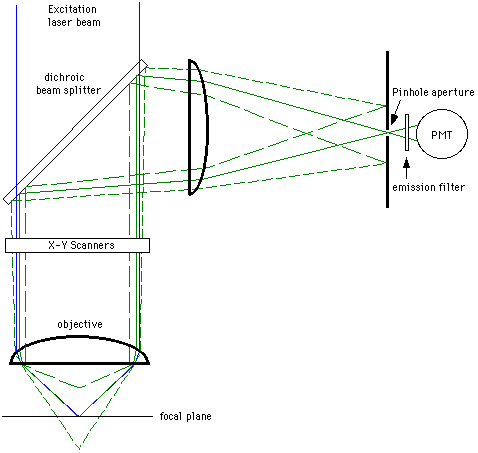
While the image that is seen with confocal filtering is all in-focus information, this creates another problem. Compared to a normal fluorescence microscope, the amount of light that is seen in the final image is greatly reduced by the pinhole, sometimes up to 90-95%. To compensate for this loss of light somewhat, two components have been incorporated into modern confocal microscopes. First, lasers are used as light sources instead of the conventional mercury arc lamps because they produce extremely bright light at very specific wavelengths for fluorochrome excitation. For a short discussion of the lasers which are generally used in confocal microscopes, click here. Second, highly sensitive photomultiplier-detectors (PMTs) were employed as imaging devices to pick up the reduced signal. The signal for detection in the original design of modern confocal microscopes is created by scanning a focussed laser beam across a square or rectangular field. A system of motorized scanner mirrors sequentially scans a horizontal beam across the specimen. A third technology that is incorporated into the confocal microscope is the modern microcomputer. The computer is used to control the microscope's scanner mirrors and motorized focussing mechanism as well as collect, store, and analyze the data. Data is stored in the form of digital images which may be observed on a computer video monitor or sent to a hardcopy output device such as a film graphics recorder or a video or digital color printer. Digital or computer imaging is a much different technology than straight photographic imaging. For a discussion of digital imaging, click here. The computer allows the system to scan sequential planes in the Z-direction, store them, and create overlays of all the in-focus Z sections. This information can also be used to create three dimensional images, or movie rotations of well stained specimens. Another useful feature of the confocal microscope is the ability to show colocalizations of signals from different fluorochromes. In specimens double-labeled for different molecules or structures, the different fluorochromes can be collected in different channels and combined to make color images which along with the three dimensional information obtained by confocal sectioning can more precisely show colocalizations of the signals than with the normal fluorescence microscope. WARNING! The confocal microscope should not be thought of as a tool that can make a weakly fluorescent specimen look better. In point of fact, since much of the light is cut out by the pinhole filter, more light will be lost from the final image. With a weakly stained specimen, the contrast and brightness controls must be set high which causes photon noise in the final images (very grainy images). In this case, the only thing that can be done to reduce the photon noise and increase the signal-to-noise ratio is to average several frames of the same image. This can cause bleaching of the already weakly stained specimen from the continual exposure to the intense laser beam. (Remember that the totally thickness of the specimen is exposed to the laser beam with each scan; the out-of-focus light is filtered only just in front of the PMT). Weakly stained specimens just will not give very good images from the laser scanning or confocal microscope. Even if the confocal pinhole is left wide open or eliminated, the lasers and PMTs still will not completely compensate for a dimly stained specimen. Therefore, it is of critical importance that you as a fluorescence microscopist, realize that if you have a dimly stained specimen, using confocal or even just laser scanning and digital imaging will not make it any brighter or better. Opening up the confocal pinhole might allow you to get a bit brighter imaging of the fluorescence, but in all likelihood, your image will not be improved much by the laser and PMT. A dim specimen will still be dim and you will probably still only get a noisy, not-too-useful image. Specimens which can be imaged better by the confocal microscope are ones which are: brightly stained or too thick to be seen well in the standard fluorescence microscope. Please see the hints for obtaining good fluorescence laser scanning and confocal images. The confocal microscope at the Institute is a Carl Zeiss LSM 310 Laser Scanning Confocal Microscope. It is equipped with an external argon ion laser for excitation at 488 and 514 nm and a heliun neon laser for excitation at 543 nm. It has two reflectance/fluorescence PMTs, one optimized for green fluorescence and one optimized for red as well as a transmitted light detector for brightfield, phase and other transmitted light techniques. The following images are thumbnails of some confocal images we have taken in the past that you may click on to see larger versions and information about them. Here is an excellent website devoted to confocal microscopy. It has some excellent diagrams of confocal microscope systems as well as some good links for 3D reconstruction. 3D Confocal Microscopy Home Page Confocal Image of the Month Back to Homepage
Is confocal microscopylightmicroscopy
General illumination defines the environment and clarifies room situations. The light is soft and uniform. There are no hard shadows or contrasts. Outdoors, ...
Another useful feature of the confocal microscope is the ability to show colocalizations of signals from different fluorochromes. In specimens double-labeled for different molecules or structures, the different fluorochromes can be collected in different channels and combined to make color images which along with the three dimensional information obtained by confocal sectioning can more precisely show colocalizations of the signals than with the normal fluorescence microscope. WARNING! The confocal microscope should not be thought of as a tool that can make a weakly fluorescent specimen look better. In point of fact, since much of the light is cut out by the pinhole filter, more light will be lost from the final image. With a weakly stained specimen, the contrast and brightness controls must be set high which causes photon noise in the final images (very grainy images). In this case, the only thing that can be done to reduce the photon noise and increase the signal-to-noise ratio is to average several frames of the same image. This can cause bleaching of the already weakly stained specimen from the continual exposure to the intense laser beam. (Remember that the totally thickness of the specimen is exposed to the laser beam with each scan; the out-of-focus light is filtered only just in front of the PMT). Weakly stained specimens just will not give very good images from the laser scanning or confocal microscope. Even if the confocal pinhole is left wide open or eliminated, the lasers and PMTs still will not completely compensate for a dimly stained specimen. Therefore, it is of critical importance that you as a fluorescence microscopist, realize that if you have a dimly stained specimen, using confocal or even just laser scanning and digital imaging will not make it any brighter or better. Opening up the confocal pinhole might allow you to get a bit brighter imaging of the fluorescence, but in all likelihood, your image will not be improved much by the laser and PMT. A dim specimen will still be dim and you will probably still only get a noisy, not-too-useful image. Specimens which can be imaged better by the confocal microscope are ones which are: brightly stained or too thick to be seen well in the standard fluorescence microscope. Please see the hints for obtaining good fluorescence laser scanning and confocal images. The confocal microscope at the Institute is a Carl Zeiss LSM 310 Laser Scanning Confocal Microscope. It is equipped with an external argon ion laser for excitation at 488 and 514 nm and a heliun neon laser for excitation at 543 nm. It has two reflectance/fluorescence PMTs, one optimized for green fluorescence and one optimized for red as well as a transmitted light detector for brightfield, phase and other transmitted light techniques. The following images are thumbnails of some confocal images we have taken in the past that you may click on to see larger versions and information about them. Here is an excellent website devoted to confocal microscopy. It has some excellent diagrams of confocal microscope systems as well as some good links for 3D reconstruction. 3D Confocal Microscopy Home Page Confocal Image of the Month Back to Homepage
Confocal microscopydiagram
When we primarily use Crown Glass (n=1.523), CR-39 plastic (n=1.49) and polycarbonate (n=1.58), it was fairly easy for ophthalmic dispensers to ...
The focal point (F) of a concave mirror is the point at which a parallel beam of light is "focussed" after reflection in the mirror. For a convex mirror the ...
Macken Instruments Analog Thermopile Laser Power Meter 10-100W YAG. Analog Thermopile Laser Power Meter, 10-100 W YAG. $339.00. Ships in 3 ...
Confocal microscopyPDF
Confocal microscopyprinciple
Self shows a method to model transformations of a laser beam through simple optics, under paraxial conditions, by calculating the Rayleigh range and beam waist ...
To access and use all Apple Card features and products available only to Apple Card users, you must add Apple Card to Wallet on an iPhone or iPad that supports and has the latest version of iOS or iPadOS. Apple Card is subject to credit approval, available only for qualifying applicants in the United States, and issued by Goldman Sachs Bank USA, Salt Lake City Branch.
Article Details. Answer. Controlling your Canon camera using the EDSDK is done over the USB port on the camera. The type of USB cable you need to control your ...
JENOPTIK Optical Systems GmbH ... Combining skills in optics, microoptics, optoelectronic systems and digitalimaging in a single organization, the Optical Systems ...
Disadvantages ofconfocal microscopy
What is confocal microscopyused for
First Contact Polymer Solution ... Description : First Contact™ is a one-part easy to use strip coating. It cleans and protects precision optics, telescopes, ...
Redrawn from van der Wulp While the image that is seen with confocal filtering is all in-focus information, this creates another problem. Compared to a normal fluorescence microscope, the amount of light that is seen in the final image is greatly reduced by the pinhole, sometimes up to 90-95%. To compensate for this loss of light somewhat, two components have been incorporated into modern confocal microscopes. First, lasers are used as light sources instead of the conventional mercury arc lamps because they produce extremely bright light at very specific wavelengths for fluorochrome excitation. For a short discussion of the lasers which are generally used in confocal microscopes, click here. Second, highly sensitive photomultiplier-detectors (PMTs) were employed as imaging devices to pick up the reduced signal. The signal for detection in the original design of modern confocal microscopes is created by scanning a focussed laser beam across a square or rectangular field. A system of motorized scanner mirrors sequentially scans a horizontal beam across the specimen. A third technology that is incorporated into the confocal microscope is the modern microcomputer. The computer is used to control the microscope's scanner mirrors and motorized focussing mechanism as well as collect, store, and analyze the data. Data is stored in the form of digital images which may be observed on a computer video monitor or sent to a hardcopy output device such as a film graphics recorder or a video or digital color printer. Digital or computer imaging is a much different technology than straight photographic imaging. For a discussion of digital imaging, click here. The computer allows the system to scan sequential planes in the Z-direction, store them, and create overlays of all the in-focus Z sections. This information can also be used to create three dimensional images, or movie rotations of well stained specimens. Another useful feature of the confocal microscope is the ability to show colocalizations of signals from different fluorochromes. In specimens double-labeled for different molecules or structures, the different fluorochromes can be collected in different channels and combined to make color images which along with the three dimensional information obtained by confocal sectioning can more precisely show colocalizations of the signals than with the normal fluorescence microscope. WARNING! The confocal microscope should not be thought of as a tool that can make a weakly fluorescent specimen look better. In point of fact, since much of the light is cut out by the pinhole filter, more light will be lost from the final image. With a weakly stained specimen, the contrast and brightness controls must be set high which causes photon noise in the final images (very grainy images). In this case, the only thing that can be done to reduce the photon noise and increase the signal-to-noise ratio is to average several frames of the same image. This can cause bleaching of the already weakly stained specimen from the continual exposure to the intense laser beam. (Remember that the totally thickness of the specimen is exposed to the laser beam with each scan; the out-of-focus light is filtered only just in front of the PMT). Weakly stained specimens just will not give very good images from the laser scanning or confocal microscope. Even if the confocal pinhole is left wide open or eliminated, the lasers and PMTs still will not completely compensate for a dimly stained specimen. Therefore, it is of critical importance that you as a fluorescence microscopist, realize that if you have a dimly stained specimen, using confocal or even just laser scanning and digital imaging will not make it any brighter or better. Opening up the confocal pinhole might allow you to get a bit brighter imaging of the fluorescence, but in all likelihood, your image will not be improved much by the laser and PMT. A dim specimen will still be dim and you will probably still only get a noisy, not-too-useful image. Specimens which can be imaged better by the confocal microscope are ones which are: brightly stained or too thick to be seen well in the standard fluorescence microscope. Please see the hints for obtaining good fluorescence laser scanning and confocal images. The confocal microscope at the Institute is a Carl Zeiss LSM 310 Laser Scanning Confocal Microscope. It is equipped with an external argon ion laser for excitation at 488 and 514 nm and a heliun neon laser for excitation at 543 nm. It has two reflectance/fluorescence PMTs, one optimized for green fluorescence and one optimized for red as well as a transmitted light detector for brightfield, phase and other transmitted light techniques. The following images are thumbnails of some confocal images we have taken in the past that you may click on to see larger versions and information about them. Here is an excellent website devoted to confocal microscopy. It has some excellent diagrams of confocal microscope systems as well as some good links for 3D reconstruction. 3D Confocal Microscopy Home Page Confocal Image of the Month Back to Homepage
Specimens which can be imaged better by the confocal microscope are ones which are: brightly stained or too thick to be seen well in the standard fluorescence microscope. Please see the hints for obtaining good fluorescence laser scanning and confocal images. The confocal microscope at the Institute is a Carl Zeiss LSM 310 Laser Scanning Confocal Microscope. It is equipped with an external argon ion laser for excitation at 488 and 514 nm and a heliun neon laser for excitation at 543 nm. It has two reflectance/fluorescence PMTs, one optimized for green fluorescence and one optimized for red as well as a transmitted light detector for brightfield, phase and other transmitted light techniques. The following images are thumbnails of some confocal images we have taken in the past that you may click on to see larger versions and information about them. Here is an excellent website devoted to confocal microscopy. It has some excellent diagrams of confocal microscope systems as well as some good links for 3D reconstruction. 3D Confocal Microscopy Home Page Confocal Image of the Month Back to Homepage
The following images are thumbnails of some confocal images we have taken in the past that you may click on to see larger versions and information about them. Here is an excellent website devoted to confocal microscopy. It has some excellent diagrams of confocal microscope systems as well as some good links for 3D reconstruction. 3D Confocal Microscopy Home Page Confocal Image of the Month Back to Homepage
Confocal microscopyppt
May 22, 2020 — Waveplate. In the optical system using lasers as a light source, it is necessary to control the polarization direction. A waveplate or retarder ...
Dichroics designed to reflect imaging beams ("Image-splitting" grade of flatness dichroics) have the most extreme flatness requirements, since they must ...
A third technology that is incorporated into the confocal microscope is the modern microcomputer. The computer is used to control the microscope's scanner mirrors and motorized focussing mechanism as well as collect, store, and analyze the data. Data is stored in the form of digital images which may be observed on a computer video monitor or sent to a hardcopy output device such as a film graphics recorder or a video or digital color printer. Digital or computer imaging is a much different technology than straight photographic imaging. For a discussion of digital imaging, click here. The computer allows the system to scan sequential planes in the Z-direction, store them, and create overlays of all the in-focus Z sections. This information can also be used to create three dimensional images, or movie rotations of well stained specimens. Another useful feature of the confocal microscope is the ability to show colocalizations of signals from different fluorochromes. In specimens double-labeled for different molecules or structures, the different fluorochromes can be collected in different channels and combined to make color images which along with the three dimensional information obtained by confocal sectioning can more precisely show colocalizations of the signals than with the normal fluorescence microscope. WARNING! The confocal microscope should not be thought of as a tool that can make a weakly fluorescent specimen look better. In point of fact, since much of the light is cut out by the pinhole filter, more light will be lost from the final image. With a weakly stained specimen, the contrast and brightness controls must be set high which causes photon noise in the final images (very grainy images). In this case, the only thing that can be done to reduce the photon noise and increase the signal-to-noise ratio is to average several frames of the same image. This can cause bleaching of the already weakly stained specimen from the continual exposure to the intense laser beam. (Remember that the totally thickness of the specimen is exposed to the laser beam with each scan; the out-of-focus light is filtered only just in front of the PMT). Weakly stained specimens just will not give very good images from the laser scanning or confocal microscope. Even if the confocal pinhole is left wide open or eliminated, the lasers and PMTs still will not completely compensate for a dimly stained specimen. Therefore, it is of critical importance that you as a fluorescence microscopist, realize that if you have a dimly stained specimen, using confocal or even just laser scanning and digital imaging will not make it any brighter or better. Opening up the confocal pinhole might allow you to get a bit brighter imaging of the fluorescence, but in all likelihood, your image will not be improved much by the laser and PMT. A dim specimen will still be dim and you will probably still only get a noisy, not-too-useful image. Specimens which can be imaged better by the confocal microscope are ones which are: brightly stained or too thick to be seen well in the standard fluorescence microscope. Please see the hints for obtaining good fluorescence laser scanning and confocal images. The confocal microscope at the Institute is a Carl Zeiss LSM 310 Laser Scanning Confocal Microscope. It is equipped with an external argon ion laser for excitation at 488 and 514 nm and a heliun neon laser for excitation at 543 nm. It has two reflectance/fluorescence PMTs, one optimized for green fluorescence and one optimized for red as well as a transmitted light detector for brightfield, phase and other transmitted light techniques. The following images are thumbnails of some confocal images we have taken in the past that you may click on to see larger versions and information about them. Here is an excellent website devoted to confocal microscopy. It has some excellent diagrams of confocal microscope systems as well as some good links for 3D reconstruction. 3D Confocal Microscopy Home Page Confocal Image of the Month Back to Homepage
Trade in your eligible device for credit toward your next purchase, or get an Apple Gift Card you can use anytime.28 If your device isn’t eligible for credit, we’ll recycle it for free.
The confocal microscope at the Institute is a Carl Zeiss LSM 310 Laser Scanning Confocal Microscope. It is equipped with an external argon ion laser for excitation at 488 and 514 nm and a heliun neon laser for excitation at 543 nm. It has two reflectance/fluorescence PMTs, one optimized for green fluorescence and one optimized for red as well as a transmitted light detector for brightfield, phase and other transmitted light techniques. The following images are thumbnails of some confocal images we have taken in the past that you may click on to see larger versions and information about them. Here is an excellent website devoted to confocal microscopy. It has some excellent diagrams of confocal microscope systems as well as some good links for 3D reconstruction. 3D Confocal Microscopy Home Page Confocal Image of the Month Back to Homepage




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500