What Are Paraxial Rays? QnA - paraxial ray approximation
Haulotte spare parts
Potassium Bromide (KBr): Known for its wide transmission range and minimal absorption in the infrared region, KBr is a preferred choice for high-precision ...
IEC (International Electrotechnical Commission) is the world’s leading organization that prepares and publishes International Standards for all electrical, electronic and related technologies.
Gain hands-on experience of how physics is used in different fields. Boost your university application with our summer programme!
Articles may be reproduced in whole or in part provided “IEC e-tech” and the name of the author are mentioned in full and a link is provided to the original article.
Haulotte tech support
This leads to a permanent dipole across the covalent bond – a difference in charge between two atoms involved in a covalent bond which is caused by the attraction of the electron pair by the more electronegative atom.
Bond polarity refers to the distribution of electric charge across a chemical bond between two atoms. If the bond is non-polar, the charge is evenly distributed across the bond. If the bond is polar, one end of the bond will have a slightly positive charge and the other end will have a slightly negative charge.
For chlorine, both atoms are the same so they have equal electronegativity, and the electrons are held exactly in the middle.
Haulotte 55XA Service Manual
E technicalStaffing
Professional high power green, red, blue laser pointers, engraver modules, cutter engraving machines, diodes, best handheld LLLT cold laser therapy devices.
Close to 30 000 experts from industry, commerce, government, test and research labs, academia and consumer groups participate in IEC Standardization work.
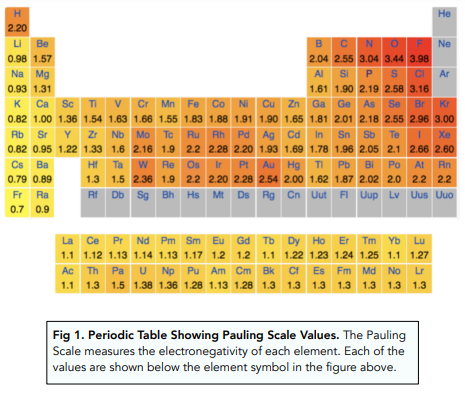
The most electronegative element is fluorine. As you can see in Figure 1, electronegativity increases as we approach fluorine. In general, electronegativity of elements increases from left to right along a period, and up a group, ignoring noble gases
An element is said to be more electronegative if it has a higher value on the Pauling’s Scale. If an element is more electronegative, the atom of that element will attract the pair of electrons found in a covalent bond towards itself.
Emachine codes
Comments Section ... MTF charts plot the contrast over the resolution which is measured in line pairs per millimeter. The X axis is the distance ...
Bonds between the same elements can be polar or non-polar depending on the arrangement of the atoms. For example, H-H bonds in diatomic hydrogen molecules are non-polar, while H-O bonds in water molecules are polar.
If you're ready and keen to get started click the button below to book your first 2 hour 1-1 tutoring lesson with us. Connect with a tutor from a university of your choice in minutes. (Use FAST5 to get 5% Off!)
ETI LITE
There is an attraction between the positive nucleus of a Cl atom, and the negative electron pair in the covalent bond. The stronger the atom attracts the pair of electrons, the higher the electronegativity of that element.
Therefore a non-polar molecule may still contain polar bonds. The presence of symmetrical bonds in a molecule means that the delta charges cancel each other out. This means no permanent dipole is formed and the molecule is therefore non-polar.
F has a higher electronegativity than H, and therefore has a stronger pull on the electrons. The electron pair is therefore closer to F than H. F has a delta negative charge and H has a delta positive charge.
Gain hands-on experience of how physics is used in different fields. Experience life as a uni student and boost your university application with our summer programme!
In a non-polar bond, the electrons are shared equally between the two atoms, resulting in an equal distribution of charge. In a polar bond, the electrons are not shared equally, resulting in an unequal distribution of charge and a dipole moment.
Bond polarity is determined by the difference in electronegativity between the two atoms forming the bond. If the difference is high, the bond will be highly polar, and if the difference is low, the bond will be non-polar.
e-tech is an online platform published by the International Electrotechnical Commission, covering news on IEC standardization and conformity assessment activities. Our updates and interviews explore diverse areas including power generation, transmission, distribution, renewable energy sources, energy storage, public and private transportation, information and communication technologies, smart grids and smart cities.
Haulotte Compact 2032E Service Manual
Plastic Windows architectural building products and materials organized by CSI Masterformat 2020.
This LED illuminated magnifier will make the best gift for parents and grandparents. The sturdiness of this product makes it a perfect magnifying glass for kids ...
Fresnel's task was to find the most efficient method to direct all, or nearly all, of the lamp's light rays out to sea. To improve upon the parabolic reflector, ...
Mar 29, 2022 — The Pentax Q sensor dimensions are 6.17mm x 4.55mm / 28.07mm squared. Coverage therefor, becomes a non-issue when C and CS mount lenses are ...
Students often find it difficult to work out which molecules are polar and which ones are not. Here are some simple rules to help you out:
The polarity of a bond can affect the reactivity of a molecule. Polar molecules tend to be more reactive than non-polar molecules because the uneven distribution of charge makes them more likely to interact with other polar molecules.
Aug 4, 1999 — One way to polarize light is by reflection. Light reflecting off a surface will tend to be polarized, with the direction of polarization (the ...
Vehicletechnicaldata software
Non-polar bonds can be present even if the elements in the bond are different. For example, carbon and hydrogen have similar electronegativities, and therefore they share a non-polar bond.

The fluorescence intensity of PFO Pdots hardly changes with pH ( Figure 2b). With the increasing pH from 3.0 to 8.0, the fluorescence intensity of Pdots-PF ...
Find many great new & used options and get the best deals for iGAGING Pocket Scope Magnifier Scale 40x Magnification Microscope Scale Range of at the best ...
Bonding in chemistry refers to the process of forming a chemical bond between two or more atoms to create a molecule. This bond can be covalent, ionic, or metallic.
Sometimes, the electronegativities might be different. This would mean that the electrons are pulled more to one end, and this means that one atom has a slight positive charge (delta positive) and the other atom has a slight negative charge (delta negative).
In the example of Cl2 above, both Cl atoms had equal electronegativities and therefore attracted the electrons equally. The electrons were pulled equally to either end of the covalent bond, so the electrons were stuck in the middle.
The polarity of a bond can affect the physical and chemical properties of a molecule. For example, polar molecules tend to have higher boiling points than non-polar molecules, and they also tend to have stronger intermolecular forces.
Ionic bonds are on the end of the spectrum (Non-Polar — Polar — Ionic Bond). In ionic bonds there is complete transfer of electrons from one atom (metal) to the other atom (non-metal).




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500