Today's NYT 'Connections' Help, Hints And Answers For ... - camera brands connections
What is raman spectroscopyand how does it work

Raman spectroscopy is a non-destructive chemical analysis technique which offers detailed information regarding chemical structure, phase and polymorphic crystallinity, and molecular interactions. It is ideal for sample identification and quantification processes.
What is raman spectroscopyin chemistry
Raman spectroscopy works by shining a monochromatic light source—usually a laser—onto a sample and detecting the scattered light. Most scattered light is at the same frequency as the excitation source and does not offer helpful information, this is known as either Rayleigh or elastic scattering.
Raman spectrums usually depict a distinct chemical fingerprint for a specific molecule or material which can identify the material or distinguish it from others quickly. Alongside mapping (or imaging) Raman systems, image creation is based on the sample’s Raman spectrum. These images display distribution of individual chemical components, polymorphs and phases, and variation in crystallinity.
Raman spectroscopyinstrumentation
Raman spectra usually plot according to the laser frequency, meaning the Rayleigh band falls between 0 cm-1. On this scale, the band positions sit at the frequencies corresponding to the energy levels of varying functional group vibrations.
Raman spectroscopy gives insight into the chemical structure and identity of a material as well as its phase and polymorphism, intrinsic stress/strain and contamination and impurity. Raman spectroscopy also employs both qualitative and quantitative applications. Spectra are specific and chemical identifications that carry out by using search algorithms against digital databases. Band areas are proportional to concentration, meaning Raman spectroscopy is amenable to straightforward quantitative analysis.
What is raman spectroscopyused for
A small amount of the scattered light shifts in energy from the laser frequency because of interactions between the incident electromagnetic waves and the vibrational energy levels of the molecules in the sample. Plotting the intensity of the shifted light against the frequency produces a Raman spectrum of the sample.
What is raman spectroscopyprinciple
Raman spectroscopy use is often in art and archeology to characterize pigments, ceramics and gemstones. It’s use is also in geology to identify minerals and their distribution, fluid inclusions and phase transitions. In addition, raman spectroscopy is useful for disease diagnoses, ensuring uniformity and component distribution in pharmaceuticals and monitoring chemical reactions.
The use of raman spectroscopy is often in in microscopic analysis, with a spatial resolution in the order of 0.5-1 µm using a Raman microscope. The Raman microscope combines a Raman spectrometer with a standard microscope. It enables high magnification visualization of a sample and Raman analysis using a microscopic laser spot.
Raman spectroscopy is also useful for the analysis of micron size particles of volumes. It can also be used to analyze individual layers within a multilayered sample. It then pinpoints contaminants and features under the surface of a transparent sample.
Raman spectroscopy is frequently used for the analysis of solids, powders, liquids, gels, inorganic/organic and biological materials, pure chemicals, mixtures and solutions. In addition, it is used for metallic oxides and their corrosions.
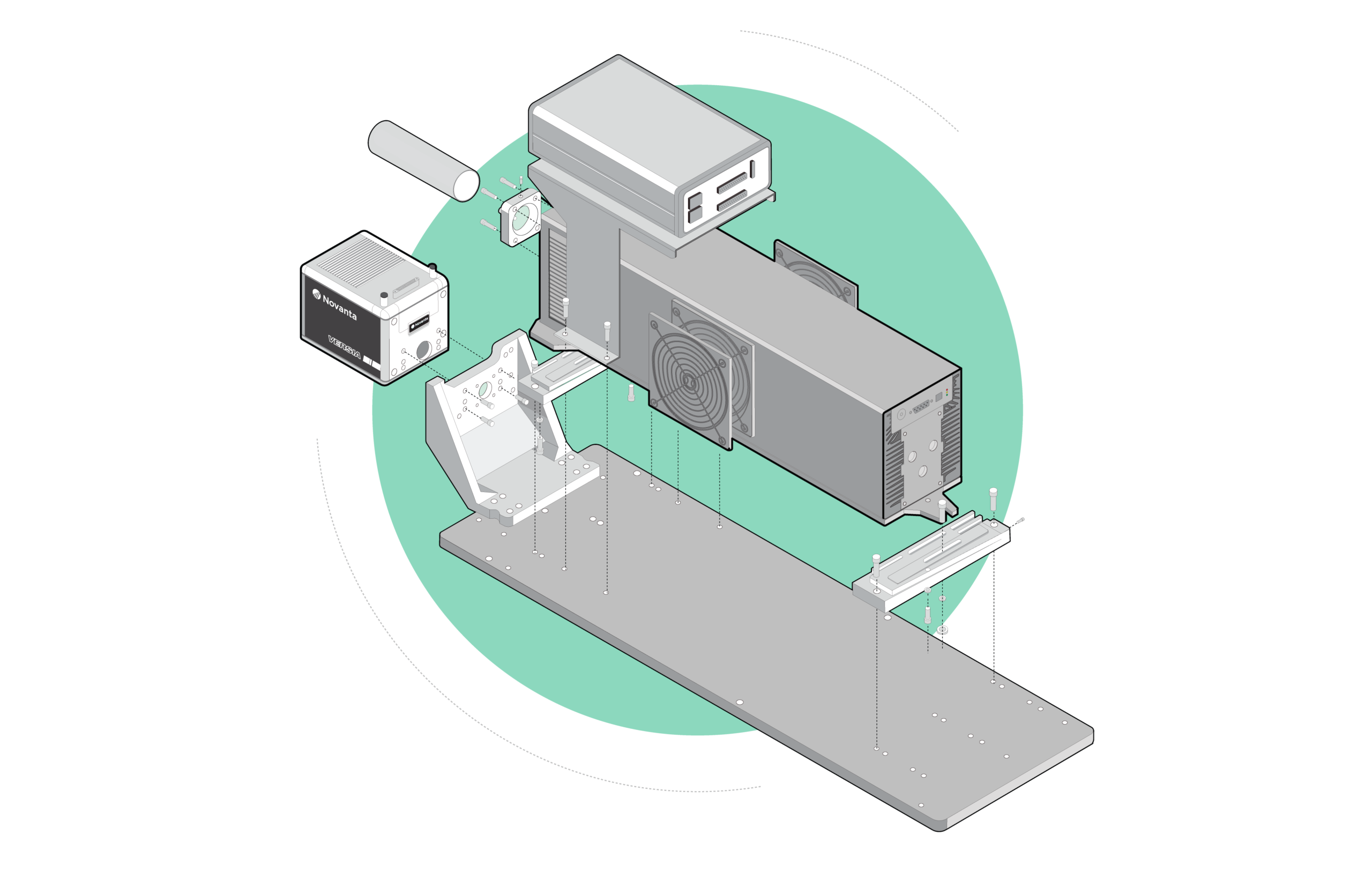
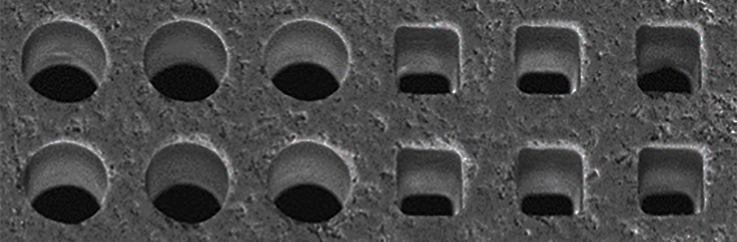
Rare-earth-doped oxide crystals such as Nd:YAG are often used as the gain medium in solid state lasers because they produce a collection of sharp emission peaks, some of which have strong gain. Rather than the typical bulk form of these crystals, thin films with planar waveguide geometry are promising alternatives for compact devices with lower lasing thresholds and better heat extraction. Such benefits motivated the growth of these oxide films by molecular beam epitaxy, a technique capable of films with precise composition, thickness and structure due to its independently controlled elemental sources. Two sets of material systems were attempted, Al-Ga-O and Y-Al-O, with Nd as the sole dopant. The films were grown on sapphire substrates of various orientations, then analyzed by x-ray diffraction and photoluminescence where peaks signaled long-range and short-range order respectively. Work on the Al-Ga-O system yielded Nd:α-Al₂O₃ (Nd:sapphire) and Nd:α-Ga₂O₃, two new laser crystals with unique collections of sharp emission peaks only observable from single-crystal films grown at temperatures far below the melting point. These conditions were required to confine the much-larger Nd dopants into Al and Ga sites with short-range order, and are unlikely reproducible by bulk methods. Due to their shared corundum structure, the emission spectra from Nd:sapphire and Nd:α-Ga₂O₃ appear similar but wavelength-shifted. The dominant Nd:sapphire peak has strong gain comparable to its counterpart from Nd:YVO₄, one of the highest available, while the blue-shifted Nd:α-Ga₂O₃ peak is likewise comparable to Nd:YAG. Alloying both produced corundum-structure crystals with peaks that shifted linearly with unit cell volume, which in turn depended on Ga/Al ratio and film stress. These Al-Ga-O crystals are promising for applications involving compositional-tuning and graded-index layers. Ternary Y-Al-O is not tunable because it has three stable phases YAM, YAP and YAG with different compositions, structures and thus emission spectra. Single-phase films of each phase were grown without single-crystal structure but still yielded sharp peaks consistent with their bulk counterparts. Short-range order was achievable because the Nd dopants were size-compatible with the Y sites. While the peaks did not shift with Y/Al ratio, peak sharpness improved when the ratio approached bulk stoichiometric values.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500