Glass polarizer D 80 mm - ovio-optics - glass polarizer
Vessel Area Density – a unitless measure that reflects the proportion of the OCT angiogram that is occupied by vessels of all caliber. This is typically accomplished by first binarizing the image such that the area occupied by vessels is comprised of white pixels and the avascular area is comprised of black pixels. A proportion of the white pixels divided by the total pixels in the image provides a measure of vessel density.
OCTmachine
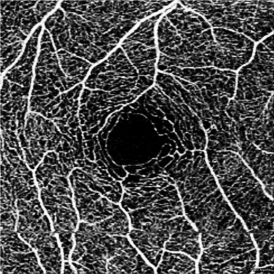
Extravascular signal from exudative edema - macular edema may appear as hypo- or hyper-reflective on structural OCTs. Hyper-reflective fluid, a form of exudate, is thought to be caused by suspended particles. OCT-A devices may capture the motion of these particles, leading to extravascular artifact. Transudative macular edema, in contrast, will not appear on OCT angiograms.
Vessel Skeletal Density – One limitation of vessel area density is the variability of vessel caliber among OCT angiograms in different eyes. For example, one eye may by chance have a greater number of larger vessels than another within a set window, thereby giving a false impression that it has increased density. Vessel skeletal density adjust for this variability by iteratively deleting outer pixels of each vessel such that each individual vessel, regardless of size, is represented by only a single line of pixels.
One asset of this OCT-based approach is that it provides a quantitative analysis of the retinal vessels (in addition to the qualitative analysis done on standard angiography). Moreover, and contrary to the "2-D" conventional angiograms, OCT-A technology provides "3-D" imaging information of the macula and visualizes peripapillary capillaries that supply the retinal nerve fiber layer.[6]
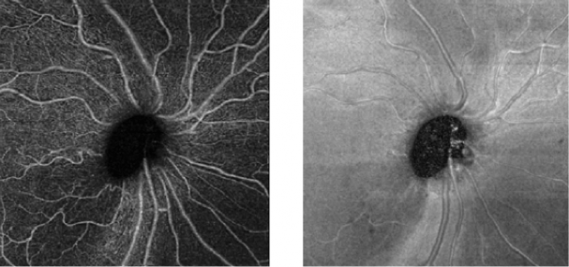
OCT-A technology uses laser light reflectance of the surface of moving red blood cells to accurately depict vessels through different segmented areas of the eye, thus eliminating the need for intravascular dyes.[2] The OCT scan of a patient's retina consists of multiple individual A-scans, which when compiled into a B-scan provides cross-sectional structural information. With OCT-A technology, the same tissue area is repeatedly imaged and differences are analyzed between scans (over time), thus allowing one to detect zones containing high flow rates (i.e. with marked changes between scans) and zones with slower, or no flow at all, which will be similar among scans.[3]
Optical coherence tomography
The detection and evaluation of choroidal vascular membranes may be similarly achieved with OCT-A in uveitis, as in the other causes referred above.[28]Specifically in inflammatory conditions, OCT-A has the advantage of acquiring three-dimensional data, and potentially improving our understanding of the pathophysiology of these diseases as well as their follow-up and management. However, multimodal imaging is still the option of choice in the diagnosis and management of uveitis.[8]
OCTprinciple
OCTin Cardiology
Light is emitted through either a spectral domain OCT (SD-OCT), with a wavelength of near 800nm; or a swept-source OCT (SS-OCT), which utilizes a longer wavelength, close to 1050nm. Longer wavelengths have a deeper tissue penetrance, but a slightly lower axial resolution. OCT-A employs two methods for motion detection: amplitude decorrelation or phase variance. The former detects differences in amplitude between two different OCT B-scans. Phase variance is related to the emitted light wave properties, and the variation of phase when it intercepts moving objects. To improve visualization and reduce background noise from normal small eye movements, two averaging methods - split spectrum amplitude decorrelation technique and volume averaging - were developed.[4][5] These OCT-A algorithms produce an image (3mm2 to 12mm2) that is segmented, by standard, into four zones: the superficial retinal plexus, the deep retinal plexus, the outer retina and the choriocapillaris. Applied to the optic disc it includes its full depth.[6][7]
Measurements are taken based on guidelines in the EMVA 1288 standard; the full definition can be found at EMVA.org. Camera settings are: maximum bit depth, 16-bit pixel format, and ISP disabled. The center wavelength is 525 nm unless otherwise noted. Results are captured at room temperature (20°C). Using FLIR test software version 4.1.
OCTeye test results
It may also prove useful as a tool for ocular blood flow research and thus to help uncover non-IOP related mechanisms in this disease.
Vessel Diameter Index – this value reflects the average vessel diameter within an OCT angiogram and is calculated by dividing vessel area density by vessel skeletal density.
However, some limitations and artifacts are important to consider in the interpretation of OCT-A images (see section below).
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Therefore, only a brief description of current uses is available in this article and further information should be looked in the available ophthalmology journals.
OCTeye test price
While clinicians primarily utilize OCT-A to qualitatively assess retinal microvasculature, researchers in recent years have aimed to develop more quantitative approaches.[29] These include the development of several vascular metrics that aim to quantify vascular features such as density and morphology. Though not exhaustive, below are several commonly used metrics.
It has been reported as a useful tool for evaluating optic disc perfusion in glaucomatous eyes, since attenuated peripapillary and macular vessel density was detectable in pre-perimetric glaucoma patients. Therefore, there is enthusiasm about the role of OCT-A in early detection of glaucomatous damage. Moreover, the quantitative data from these retinal vessels may prove useful in analysing metabolic activity from the inner layers of the retina and thus provide further advances in monitoring function and progression in this disease.[14][24]
OCTppt
The main advantages are the shorter acquisition time and that it is a non-invasive process. Fluorescein and indocyanine-green angiography require an injectable dye (which takes time to reach retinal vessels, and may be associated with systemic adverse effects and even anaphylatic reactions[2]).
As a fast, safe and noninvasive procedure to assess the chorioretinal microvasculature, OCT-A has been increasingly used in retinal diseases. The number of studies reporting new findings and utilities is exponentially growing.
Segmentation Error - Automated segmentation of a structural abnormal retina is an unavoidable limitation of OCT-A imaging. In cases of pigment epithelial detachments (PED), one should look carefully for segmentation errors and manually edit layers if deemed necessary for a correct interpretation.[31]
OCTinterpretation PDF
Media Opacities - media opacities such as corneal scarring, cataracts, posterior capsular opacification, and vitreous floaters may lead to signal attenuation and shadowing artifact.[3] These may obscure portions of the OCT angiogram or lead to diffuse reduction in image quality.
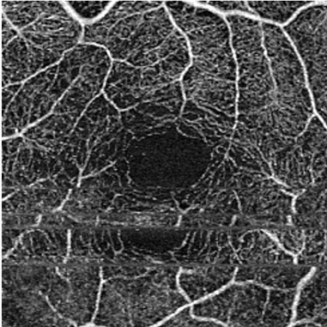
Motion Artifact - OCT-A examination is motion-sensitive and requires patients to be able to reasonably fixate, which may be difficult to obtain for the visually impaired.[11] Excessive motion of the eye can lead to motion artifacts as seen in the image. This can be overcome to some extent with a tracking feature present on most devices.
Projection Artifact - Projection artifact is inevitable and is related to light that traverses blood vessels and is reflected back by deeper layers (e.g. pigment retinal epithelium), which will appear in the final deep image with a similar vascular pattern as the overlying superficial vessels.[30]
Francesco Pichi and collaborators recently reviewed the uses and importance of OCT-A in uveitis.[8] For the sake of this article, only a brief summary will be reported.
Optical coherence tomography angiography (OCT-A) has emerged as a non-invasive technique for imaging the microvasculature of the retina and the choroid. The first clinical studies using this innovative technology were published in 2014 .[1]




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500