Dangers of Ultraviolet Radiation - infrared and ultraviolet
Pulse broadening limits the achievable data transmission rates in optical fiber communication. Higher dispersion leads to more severe pulse spreading, necessitating lower data rates to maintain reliable communication and minimize errors.
Dispersion is a crucial aspect to consider in optical fiber communication systems, as it directly impacts data transmission quality and capacity. By understanding the different types of dispersion and their effects on signal propagation, engineers can design and optimize optical fiber networks to achieve higher data rates and longer transmission distances. As technology continues to advance, research and innovation in dispersion compensation techniques will play a vital role in improving optical fiber communication and meeting the ever-increasing demands for faster and more reliable data transmission.
When we talk about modern communication systems, optical fibers play a pivotal role in facilitating high-speed data transmission over long distances. The ability to carry vast amounts of data efficiently and reliably is critical for a wide array of applications, including telecommunications, data centers, and the Internet. However, as data rates and transmission distances increase, so do the challenges in maintaining signal integrity. One of the primary factors that affect signal quality in optical fibers is dispersion. Other than that, there are other key effects of dispersion on communication which are as follows:
Dispersion is primarily caused by the varying refractive index of the fiber material, leading to different propagation speeds for different wavelengths of light. Two main types of dispersion occur, the first one is chromatic dispersion, caused by material properties, and the other one is polarization mode dispersion which occurs due to structural imperfections.
UN-NumberDOT, IMDG, IATAUN3283UN proper shipping nameDOTSelenium compound, solid, n.o.s. (Zinc selenide)IMDGSELENIUM COMPOUND, SOLID, N.O.S.(Zinc selenide), MARINE POLLUTANTIATASELENIUM COMPOUND, SOLID, N.O.S. (Zinc selenide)Transport hazard class(es)DOTClass6.1 Toxic substances.Label6.1Class6.1 (T5) Toxic substancesLabel6.1IMDGClass6.1 Toxic substances.Label6.1IATAClass6.1 Toxic substances.Label6.1Packing groupDOT, IMDG, IATAIIIEnvironmental hazards:Environmentally hazardous substance, solid; Marine PollutantMarine pollutant (IMDG):Yes (P)Symbol (fish and tree)Special precautions for userWarning: Toxic substancesEMS Number:F-A,S-ATransport in bulk according to Annex II of MARPOL73/78 and the IBC CodeN/ATransport/Additional information:DOTMarine Pollutant (DOT):NoRemarks:Special marking with the symbol (fish and tree).UN "Model Regulation":UN3283, Selenium compound, solid, n.o.s. (Zinc selenide), 6.1, III
See more Zinc products. Zinc (atomic symbol: Zn, atomic number: 30) is a Block D, Group 12, Period 4 element with an atomic weight of 65.38. The number of electrons in each of zinc's shells is 2, 8, 18, 2, and its electron configuration is [Ar] 3d10 4s2. The zinc atom has a radius of 134 pm and a Van der Waals radius of 210 pm. Zinc was discovered by Indian metallurgists prior to 1000 BC and first recognized as a unique element by Rasaratna Samuccaya in 800. Zinc was first isolated by Andreas Marggraf in 1746. In its elemental form, zinc has a silver-gray appearance. It is brittle at ordinary temperatures but malleable at 100 °C to 150 °C. It is a fair conductor of electricity, and burns in air at high red producing white clouds of the oxide. Zinc is mined from sulfidic ore deposits. It is the 24th most abundant element in the earth's crust and the fourth most common metal in use (after iron, aluminum, and copper). The name zinc originates from the German word "zin," meaning tin.
ReactivityNo data availableChemical stabilityStable under recommended storage conditions.Thermal decomposition / conditions to be avoided:Decomposition will not occur if used and stored according to specifications.Possibility of hazardous reactionsNo dangerous reactions knownConditions to avoidNo data availableIncompatible materials:BasesOxidizing agentsHazardous decomposition products:Hydrogen selenideSelenium dioxide (SeO2)Metal oxide fume
The Study of Metal Sulfide as Efficient Counter Electrodes on the Performances of CdS/CdSe/ZnS-co-sensitized Hierarchical TiO2 Sphere Quantum Dot Solar Cells.
GHS06 GHS08Signal word: DangerHazard statementsH301+H331 Toxic if swallowed or if inhaled.H373 May cause damage to the central nervous system, the liver and the digestive system through prolonged or repeated exposure.Route of exposure: Oral,Inhalative.Precautionary statementsP273 Avoid release to the environment.P270 Do not eat, drink or smoke when using this product.P309 IF exposed or if you feel unwell:P310 Immediately call a POISON CENTER or doctor/physician.P302+P352 IF ON SKIN: Wash with plenty of soap and water.P501 Dispose of contents/container in accordance with local/regional/national/international regulations.WHMIS classificationD1A - Very toxic material causing immediate and serious toxic effectsClassification systemHMIS ratings (scale 0-4)(Hazardous Materials Identification System) HEALTH FIRE REACTIVITY20 1Health (acute effects) = 2Flammability = 0Physical Hazard = 1Other hazardsResults of PBT and vPvB assessmentPBT: N/AvPvB: N/A
Additional information about design of technical systems:Properly operating chemical fume hood designed for hazardous chemicals and having an average face velocity of at least 100 feet per minute.Control parametersComponents with limit values that require monitoring at the workplace:Selenium and selenium compounds (as Se) mg/m3ACGIH TLV 0.2Austria MAK 0.1Belgium TWA 0.2Denmark TWA 0.1Finland TWA 0.1; 0.3-STELGermany MAK 0.1Hungary 0.1-STELIreland TLV 0.1Japan OEL 0.1Korea TLV 0.2Netherlands MAC-TGG 0.1Poland TWA 0.1; 0.3-STELSweden NGV 0.1Switzerland MAK-W 0.1United Kingdom TWA 0.1USA PEL 0.21315-09-9 Zinc selenide (100.0%)PEL (USA) Long-term value: 0.2 mg/m3 as SeREL (USA) Long-term value: 0.2 mg/m3 as SeTLV (USA) Long-term value: 0.2 mg/m3 as SeEL (Canada) Long-term value: 0.1 mg/m3 as SeAdditional information: No dataExposure controlsPersonal protective equipmentFollow typical protective and hygienic practices for handling chemicals.Keep away from foodstuffs, beverages and feed.Remove all soiled and contaminated clothing immediately.Wash hands before breaks and at the end of work.Store protective clothing separately.Maintain an ergonomically appropriate working environment.Breathing equipment:Use self-contained respiratory protective device in emergency situations.Protection of hands: Impervious glovesInspect gloves prior to use.Suitability of gloves should be determined both by material and quality, the latter of which may vary by manufacturer.Eye protection: Safety glassesBody protection: Protective work clothing.

Information on toxicological effectsAcute toxicity:Toxic if inhaled.Toxic if swallowed.LD/LC50 values that are relevant for classification: No dataSkin irritation or corrosion: May cause irritationEye irritation or corrosion: May cause irritationSensitization: No sensitizing effects known.Germ cell mutagenicity: No effects known.Carcinogenicity:No classification data on carcinogenic properties of this material is available from the EPA, IARC, NTP, OSHA or ACGIH.Reproductive toxicity: No effects known.Specific target organ system toxicity - repeated exposure:May cause damage to the central nervous system, the liver and the digestive system through prolonged or repeated exposure. Route of exposure: Oral, Inhalative.Specific target organ system toxicity - single exposure: No effects known.Aspiration hazard: No effects known.Subacute to chronic toxicity:Selenium may cause amyotropic lateral sclerosis, bronchial irritation, gastrointestinal distress, vasopharyngeal irritation, garlic odor on breath and sweat, metallic taste, pallor, irritability, excessive fatigue, loss of fingernails and hair, pulmonary edema, anemia and weight loss.Zinc fumes may cause metal fume fever. Effects include dry throat, metallic taste, chest pain, dyspnea, rales and dry cough.Several hours later, chills may occur with lassitude, malaise, fatigue, headache, back pain, muscle cramps, blurred vision, nausea, fever, perspiration, vomiting and leukocytosis.Subacute to chronic toxicity: No effects known.Additional toxicological information:To the best of our knowledge the acute and chronic toxicity of this substance is not fully known.Carcinogenic categoriesOSHA-Ca (Occupational Safety & Health Administration)Substance is not listed.
Personal precautions, protective equipment and emergency proceduresUse personal protective equipment. Keep unprotected persons away.Ensure adequate ventilationEnvironmental precautions:Do not allow material to be released to the environment without official permits.Methods and materials for containment and cleanup:Dispose of contaminated material as waste according to section 13.Ensure adequate ventilation.Prevention of secondary hazards:No special measures required.Reference to other sectionsSee Section 7 for information on safe handlingSee Section 8 for information on personal protection equipment.See Section 13 for disposal information.
Zinc selenideLED
HandlingPrecautions for safe handlingKeep container tightly sealed.Store in cool, dry place in tightly closed containers.Ensure good ventilation at the workplace.Open and handle container with care.Information about protection against explosions and fires:No data availableConditions for safe storage, including any incompatibilitiesRequirements to be met by storerooms and receptacles:No special requirements.Information about storage in one common storage facility:Store away from oxidizing agents.Further information about storage conditions:Keep container tightly sealed.Store in cool, dry conditions in well-sealed containers.Specific end use(s)No data available
Dispersion can distort the original shape of light pulses, resulting in signal degradation. This distortion can cause errors in data transmission, leading to the need for error correction techniques or retransmissions, reducing overall system efficiency.
Modeling and optimization of ultrasound-assisted high performance adsorption of Basic Fuchsin by starch-capped zinc selenide nanoparticles/AC as a novel composite using response surface methodology.
Information on basic physical and chemical propertiesAppearance:Form: Solid in various formsColor: RedOdor: OdorlessOdor threshold: No data available.pH: N/AMelting point/Melting range: >1100 °C (>2012 °F)Boiling point/Boiling range: No data availableSublimation temperature / start: No data availableFlash point: N/AFlammability (solid, gas): No data available.Ignition temperature: No data availableDecomposition temperature: No data availableAutoignition: No data available.Danger of explosion: Product does not present an explosion hazard.Explosion limits:Lower: No data availableUpper: No data availableVapor pressure: N/ADensity at 20 °C (68 °F): 5.42 g/cm3 (45.23 lbs/gal)Relative density: No data available.Vapor density: N/AEvaporation rate: N/ASolubility in Water (H2O): InsolublePartition coefficient (n-octanol/water): No data available.Viscosity:Dynamic: N/AKinematic: N/AOther informationNo data available
Ultraviolet/ultrasound-activated persulfate for degradation of drug by zinc selenide quantum dots: Catalysis and microbiology study.
Dispersion in optical fiber is commonly represented by the formula: Ît = (D * L) / c, where Ît is the pulse broadening, D is the dispersion coefficient, L is the fiber length, and c is the speed of light in a vacuum.
Extinguishing mediaSuitable extinguishing agentsProduct is not flammable. Use fire-fighting measures that suit the surrounding fire.Special hazards arising from the substance or mixtureIf this product is involved in a fire, the following can be released:Selenium dioxide (SeO2)Metal oxide fumeAdvice for firefightersProtective equipment:Wear self-contained respirator.Wear fully protective impervious suit.
Zinc selenideuses
To manage PMD, fiber-optic systems are designed with carefully selected materials and components that compensate for the differential delays. While these measures help, PMD can still be a concern in long-distance and high-data-rate applications, requiring constant improvements in fiber-optic technology to ensure reliable communication.

Zinc selenideformula
Polarization Mode Dispersion (PMD) is an optical issue in fiber-optic communication. When light travels through the fiber, it consists of two polarization states: horizontal and vertical. In a perfect scenario, both states would travel at the same speed, and data transmission would be seamless. However, imperfections in the fiber cause the polarization modes to travel at slightly different speeds. This leads to a time difference between the two components, causing the light pulse to spread out and arrive distorted at the receiver. As a result, data can get corrupted, reducing the signal quality and data transmission rates.
Think of it like this: Imagine a beam of white light passing through a glass prism. Instead of staying together as white light, the light splits into a rainbow of colors. This happens because each color travels at a slightly different speed inside the glass. This separation of colors is what we call dispersion.
Zinc selenidepowder
In long-distance fiber-optic links, dispersion becomes more pronounced as the signals propagate over greater distances. At some point, the accumulated dispersion can become too significant to maintain reliable communication, imposing limitations on the transmission distance.
In Wavelength Division Multiplexing systems, where multiple wavelengths are used to transmit data simultaneously, dispersion can introduce varying delays among different channels, requiring additional dispersion compensation techniques.
For example, in a step-index multimode fiber, light traveling at different angles experiences varied path lengths, causing the pulses to spread out and degrade over long distances. This issue becomes increasingly significant at higher data rates, limiting the fiber's ability to support modern high-speed communication systems.
Description of first aid measuresGeneral informationImmediately remove any clothing soiled by the product.Remove breathing apparatus only after contaminated clothing has been completely removed.In case of irregular breathing or respiratory arrest provide artificial respiration.If inhaled:Supply patient with fresh air. If not breathing, provide artificial respiration. Keep patient warm.Seek immediate medical advice.In case of skin contact:Immediately wash with soap and water; rinse thoroughly.Seek immediate medical advice.In case of eye contact:Rinse opened eye for several minutes under running water. Consult a physician.If swallowed:Do not induce vomiting; immediately call for medical help.Information for doctorMost important symptoms and effects, both acute and delayedNo data availableIndication of any immediate medical attention and special treatment neededNo data available
ToxicityAquatic toxicity:No data availablePersistence and degradabilityNo data availableBioaccumulative potentialNo data availableMobility in soilNo data availableEcotoxical effects:Remark:Very toxic for aquatic organismsAdditional ecological information:Do not allow material to be released to the environment without official permits.Do not allow product to reach groundwater, water courses, or sewage systems, even in small quantities.Danger to drinking water if even extremely small quantities leak into the ground.Also poisonous for fish and plankton in water bodies.May cause long lasting harmful effects to aquatic life.Avoid transfer into the environment.Very toxic for aquatic organismsResults of PBT and vPvB assessmentPBT: N/AvPvB: N/AOther adverse effectsNo data available
Zinc selenideblue LED
Dispersion in optical fibers is commonly measured using parameters like the dispersion coefficient (D), dispersion slope, or the material's dispersion value per unit length. Specialized equipment and techniques, such as pulse broadening measurements, are used to quantify the amount of dispersion present in the fiber.
Classification of the substance or mixtureClassification according to Regulation (EC) No 1272/2008GHS06 Skull and crossbonesAcute Tox. 3 H301 Toxic if swallowed.Acute Tox. 3 H331 Toxic if inhaled.GHS08 Health hazardSTOT RE 2 H373 May cause damage to the central nervous system, the liver and the digestive system through prolonged or repeated exposure. Route of exposure: Oral, Inhalative.Hazards not otherwise classifiedNo data availableLabel elementsLabelling according to Regulation (EC) No 1272/2008The substance is classified and labeled according to the CLP regulation.Hazard pictograms
Safety, health and environmental regulations/legislation specific for the substance or mixtureNational regulationsAll components of this product are listed in the U.S. Environmental Protection Agency Toxic Substances Control Act Chemical substance Inventory.All components of this product are listed on the Canadian Non-Domestic Substances List (NDSL).SARA Section 313 (specific toxic chemical listings)1315-09-9 Zinc selenideCalifornia Proposition 65Prop 65 - Chemicals known to cause cancerSubstance is not listed.Prop 65 - Developmental toxicitySubstance is not listed.Prop 65 - Developmental toxicity, femaleSubstance is not listed.Prop 65 - Developmental toxicity, maleSubstance is not listed.Information about limitation of use:For use only by technically qualified individuals.This product contains selenium and is subject to the reporting requirements of section 313 of the Emergency Planning and Community Right to Know Act of 1986 and 40CFR372.This product contains zinc and is subject to the reporting requirements of section 313 of the Emergency Planning and CommunityRight to Know Act of 1986 and40CFR372.Other regulations, limitations and prohibitive regulationsSubstance of Very High Concern (SVHC) according to the REACH Regulations (EC) No. 1907/2006.Substance is not listed.The conditions of restrictions according to Article 67 and Annex XVII of the Regulation (EC) No 1907/2006 (REACH) for the manufacturing, placing on the market and use must be observed.Substance is not listed.Annex XIV of the REACH Regulations (requiring Authorisation for use)Substance is not listed.Chemical safety assessment:A Chemical Safety Assessment has not been carried out.
Zinc Selenidewindow
Influence of Post-Heat Treatment of ZnO:Al Transparent Electrode for Copper Indium Gallium Selenide Thin Film Solar Cell.
Dispersion doesn't just happen with light; it can also occur with other types of waves, like sound waves or radio waves. When waves disperse, their different components move through the material at varying speeds, leading to interesting effects and colorful displays, like the rainbow produced by a prism.
To understand this better, consider a scenario where multiple light pulses of different wavelengths are transmitted through an optical fiber. Over a long distance, chromatic dispersion causes the pulses to spread out, making it challenging to distinguish individual bits of information.
Zinc Selenidelens
No, attenuation and dispersion are not the same. Attenuation refers to the loss of signal intensity as light travels through the fiber, while dispersion relates to the spreading out of light pulses due to varying propagation speeds.
In technical terms, dispersion in optical fiber refers to the phenomenon where different wavelengths of light experience varying velocities as they travel through the fiber. It causes pulses of light to spread out over time, leading to signal degradation and limiting the transmission capacity of the fiber. There are different types of dispersion in optical fibers, letâs understand them one by one.
SubstancesCAS No. / Substance Name:1315-09-9 Zinc selenideIdentification number(s):EC number: 215-259-7Index number: 034-002-00-8
Zinc selenidenanoparticles
Modal dispersion arises in multimode fibers, where light can take multiple paths or modes as it propagates through the fiber. Each mode follows a distinct path and experiences different propagation times, leading to pulse spreading. Modal dispersion becomes more pronounced in fibers with larger core diameters, reducing their capacity to transmit data accurately.
To reduce the detrimental effects of dispersion in optical fibers, various dispersion compensation techniques have been developed. The two primary approaches are dispersion-shifted fibers and dispersion-compensating fibers. Dispersion-shifted fibers are engineered to reduce the effects of chromatic dispersion by altering the fiber's dispersion profile, pushing the zero-dispersion wavelength to longer wavelengths where chromatic dispersion is less pronounced. Dispersion-compensating fibers, on the other hand, are designed to have opposite dispersion characteristics to the main transmission fiber, enabling effective compensation of dispersion.
In simple terms, dispersion is a phenomenon where different colors or components of a wave travel at different speeds through a material, causing the wave to spread out or separate.
As light signals travel through an optical fiber, dispersion causes different wavelengths to travel at different speeds, leading to pulse broadening. This spreading out of the pulses in time can cause overlapping and intersymbol interference, making it challenging for the receiver to distinguish between different bits or symbols in the data stream.
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH). The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. American Elements shall not be held liable for any damage resulting from handling or from contact with the above product. See reverse side of invoice or packing slip for additional terms and conditions of sale. COPYRIGHT 1997-2022 AMERICAN ELEMENTS. LICENSED GRANTED TO MAKE UNLIMITED PAPER COPIES FOR INTERNAL USE ONLY.
Product Number: All applicable American Elements product codes, e.g. ZN-SE-05-I , ZN-SE-05-L , ZN-SE-05-P , ZN-SE-05-ST , ZN-SE-05-WF , ZN-SE-05-PCS , ZN-SE-05-GR
This type of dispersion is caused by the spectral width of the light emitted from the transmitter (e.g., LED or laser) used in optical fiber communication. The spectral width determines the range of different wavelengths emitted. The longer wavelengths typically travel faster than the shorter ones, causing light pulses to broaden and overlap, reducing the achievable data rate.
Imagine writing a sentence, but the letters get jumbled up. This makes it harder for the receiver to read and interpret the message correctly. In the same way, dispersion in optical fiber can cause the signals to overlap and interfere with each other, making it challenging for the receiver to understand the original message. Today, we will learn in detail about dispersion, its types, and its effects on optical fiber communication.
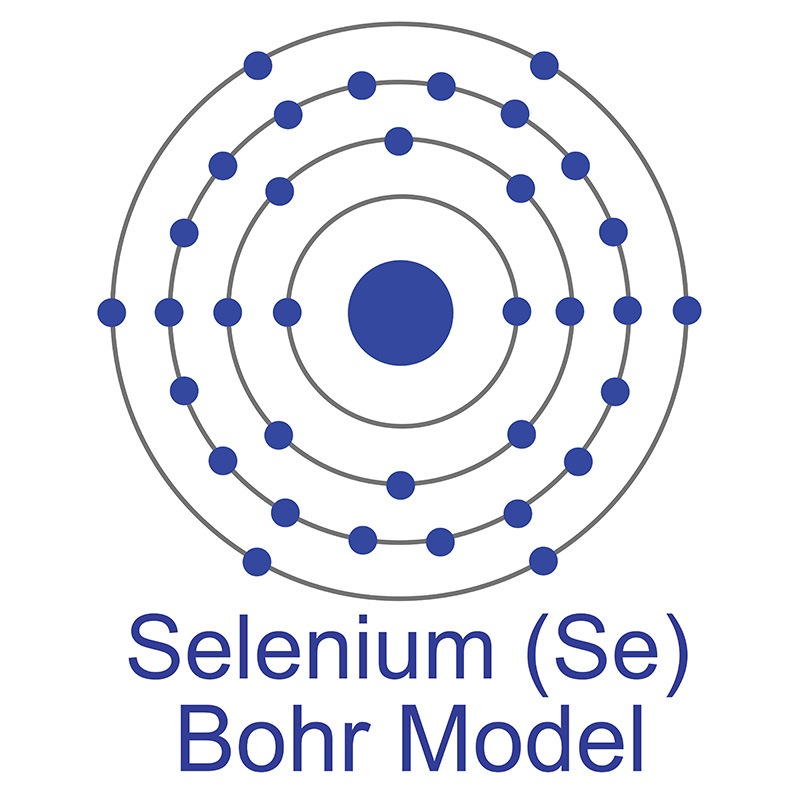
See more Selenium products. Selenium (atomic symbol: Se, atomic number: 34) is a Block P, Group 16, Period 4 element with an atomic radius of 78.96. The number of electrons in each of Selenium's shells is 2, 8, 18, 6 and its electron configuration is [Ar] 3d10 4s2 4p4. The selenium atom has a radius of 120 pm and a Van der Waals radius of 190 pm. Selenium is a non-metal with several allotropes: a black, vitreous form with an irregular crystal structure three red-colored forms with monoclinic crystal structures and a gray form with a hexagonal crystal structure, the most stable and dense form of the element. One of the most common uses for selenium is in glass production the red tint that it lends to glass neutralizes green or yellow tints from impurities in the glass materials. Selenium was discovered and first isolated by Jöns Jakob Berzelius and Johann Gottlieb Gahn in 1817. The origin of the name Selenium comes from the Greek word "Selênê," meaning moon.
Waste treatment methodsRecommendationConsult official regulations to ensure proper disposal.Uncleaned packagings:Recommendation:Disposal must be made according to official regulations.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500