Mastering M12 Connectors: A Comprehensive Guide for ... - m12 pin out
Compared to the system without back-illuminated Si photoanodes, external the voltage input was lower by 700 mV in Si photoanode-assisted flow cell, indicating that the high photovoltage can be provided by the back-illuminated Si photoanode to produce multi-carbon products (Fig. 4b). The FE for C2+ products including C2H4, C2H5OH, and propanol reaches 53% on Cu catalysts, comparable to FE of unmodified metallic copper electrocatalysts (Fig. 4c)52,53,54. Furthermore, the FE of C2+ products reached 53% with the help of Si photoanode during its 5 h operation (Fig. 4d). Therefore, the back-illuminated Si photoanode can feasibly be coupled with CO2RR flow cell operating at high reaction rates and maintain the same FE and stability as electrocatalytic CO2 reduction flow cells.
Dong, W. J. et al. CuS-decorated GaN nanowires on silicon photocathodes for converting CO2 mixture gas to HCOOH. J. Am. Chem. Soc. 143, 10099–10107 (2021).
Photoelectrochemical (PEC) reduction of CO2 use sunlight to directly drive the CO2RR for solar fuel production1,2,3. PEC CO2 reduction systems, that utilize photocathode4,5 and photoanode6,7 driven systems, have been widely investigated. Photocathodes can employ CO2RRs catalysts to carry out the CO2RR in H-type cells, where the cell voltage could be supplemented by photovoltage, thus lowering the external energy input by photovoltage generated by photoelectrode8,9,10. However, many photocathodes suffer from low activity or poor stability for PEC CO2RR, because the catalyst/semiconductor interface tends to produce hydrogen and suffers from photo-corrosion in aqueous electrolytes2,5,11,12,13,14. Furthermore, the deployment of photocathodes in flow cell configurations for PEC CO2 reduction remains a challenge because most high-performance semiconductor materials exist as dense layers, for which a compact substrate is always needed. Thus, it is a great challenge to simultaneously use semiconductors as a gas diffusion layer and sunlight absorber. On the other hand, photoanodes such as TiO215,16, WO37, BiVO47,17, GaAs18, and Si19 have been integrated to form PEC CO2 reduction cells, and exhibit a reduced cell voltage for CO2 reduction. However, there is still room to increase the current density, because many photoanode-assisted PEC CO2RR were all carried out in H-type cell20,21. The photoanode promoted flow cell for PEC CO2 reduction is a promising way to improve the energy utilization efficiency.
The thermodynamic potential of the respective product (E0) of CO, C2H4, C2H5OH, and C3H7OH are respectively equal to 1.334, 1.150, 1.147, and 1.131 V20,51. VII is the cell voltage measured in two electrodes (Figs. 3b, 4b). I is the operating current density at different voltages. FE is Faradaic efficiency for target product. Pin is the input power (100 mW/cm2 in this work). The active area of the Si photoanode was 1 cm2.
APS-C
Ros, C., Andreu, T. & Morante, J. R. Photoelectrochemical water splitting: a road from stable metal oxides to protected thin film solar cells. J. Mater. Chem. A 8, 10625–10669 (2020).
The image below shows a variety of microscope systems. There are multiple magnification objectives depicted: low power 4x, medium power, 40x and high power 100x. The distance from the end of the objective to the sample, which is called the working distance, is going to be larger on a low power objective, less on a medium power and very fine, possibly a fraction of a millimeter for the high power system. What’s critical is the angle. The sample is illuminated and light is coming out of it. In the case of the shorter working distance, higher numerical aperture objectives, that light is coming at an increasingly higher angle. The Numerical Aperture (NA) of the objective equals the sine of the half-angle (theta divided by two where theta is the entire angle).
Cofell, E. R., Nwabara, U. O., Bhargava, S. S., Henckel, D. E. & Kenis, P. J. A. Investigation of electrolyte-dependent carbonate formation on gas diffusion electrodes for CO2 electrolysis. ACS Appl. Mater. Interfaces 13, 15132–15142 (2021).
a The current-voltage curves, b ABPE and c Time-dependent photocurrents (left-axis) and Faradaic efficiency toward O2 (right-axis) under simulated AM 1.5 G illumination. Inset: Schematic of the light absorption and reaction decoupled Si photoanode.
Li, H. et al. High-rate CO2 electroreduction to C2+ products over a copper-copper iodide catalyst. Angew. Chem. Int. Ed. 60, 14329–14333 (2021).
The thickness of amorphous Si, TiO2, Ni, Au and Ag layers on polished Si(100) monitor substrate was determined by spectroscopic ellipsometer (M-2000 DI, J.A. Woollam Co., Inc.) at 60° and 70° incident angle, by fitting the amplitude ratio (Ψ) and phase shift (Δ) of polarized light with the Cauchy dispersion model for a-Si and TiO2, and a tabulated metallic model for Ni, Au, and Ag. The morphology was carried out by field emission scanning electron microscope (FESEM, Hitachi S-4800, 5 kV). XPS analysis was conducted on a Physical Electronics PHI 1600 ESCA system with an Al Kα X-ray source (1486.6 eV). The binding energy was calibrated against the C 1 s photoelectron peak at 284.6 eV as the reference. The optical reflectance measurement of the Si was performed using spectrophotometer (Shimadzu UV-3600). The X-ray diffraction (XRD) (D/MAX-2500, Rigaku) spectra were collected over a 2θ range from 20° to 80° at a scanning speed of 0.02° per step.
Covers key formulas for selecting the optimal imaging sensor and microscope objective for your digital imaging application including sensor size, magnification, field of view, pixel sizes, resolution, depth of field, and numerical aperture.
a The schematic of Ag cathode. b The J–V curves of CO2RR with and without Si photoanode in a two-electrode configuration. c The FE of CO at different current densities. d Time-dependent photocurrents (left-axis) and FE toward CO (right-axis) under simulated AM 1.5 G illumination.
Gurudayal, G. et al. Si photocathode with Ag-supported dendritic Cu catalyst for CO2 reduction. Energy Environ. Sci. 12, 1068–1077 (2019).
Optical format
To optimize the field of view for a particular application, it is vital to consider the size of the specimen and the level of detail required. For example, if a large sample with low detail is being observed, a lower magnification and larger field of view may be more appropriate. Conversely, if a smaller specimen with high detail is being observed, a higher magnification and smaller field of view may be necessary to achieve the desired level of resolution.
Yu, Y. et al. Enhanced photoelectrochemical efficiency and stability using a conformal TiO2 film on a black silicon photoanode. Nat. Energy 2, 17045 (2017).
Guo, B. et al. Facile integration between Si and catalyst for high-performance photoanodes by a multifunctional bridging layer. Nano Lett. 18, 1516–1521 (2018).
Zhong, D. et al. Coupling of Cu(100) and (110) facets promotes carbon dioxide conversion to hydrocarbons and alcohols. Angew. Chem. Int. Ed. 60, 4879–4885 (2021).
Benefiting from the back-illuminated configuration, this Si photoelectrode could be used as the photoanode for OER in the CO2RR flow cell. The CO products were generated using metallic Ag as the catalyst (photograph in Fig. S10a) alongside our back-illuminated Si photoanode in flow cell with (photograph in Figs. S11, S12). 50 nm Ag catalyst was deposited on carbon paper by DC sputtering (Fig. 3a), which appeared to a continuous surface (Fig. S13). Ag catalyst is highly crystalline and shows a face-centered cubic crystal structure (Fig. S14). The CO2RR was carried out in 1 M KOH to suppress the hydrogen evolution, and the Si photoanode was performed under AM 1.5 G illumination (100 mW/cm2). To verify the benefits of back-illuminated Si photoanode in lowering cell voltage, the current–voltage (J–V) curves of CO2RR were collected in a two-electrode configuration (Fig. 3b). The back-illuminated Si photoanode promoted flow cell only required cell voltages as low as 1.44 V to obtain the current density of 10 mA/cm2, which is 720 mV lower than the conventional dark anode (p+-Si/TiO2/Ni) using Ni as the catalyst (2.16 V). This is consistent with the photovoltage of the back-illuminated Si photoanode (Fig. 2a). Therefore, the external energy input for CO2RR was significantly decreased by the reduction OER energy consumption. The performance of the back-illuminated Si photoanode-assisted CO2RR to produce CO was evaluated over Ag catalysts at different current densities (Fig. 3c), where the exclusive formation of gaseous CO and H2 is observed in the gas phase. The Ag catalyst exhibited a selectivity of more than 90% for the reduction of CO2 to CO under wide current density range of 3–38 mA/cm2 (Fig. 3c), which is consistent with previously reported results in electrochemical CO2 reduction44,45,46. Moreover, the durability of PEC flow cell for CO production was evaluated, where the FE of CO remained nearly unchanged throughout the test duration (Fig. 3d). To further evaluate the stability of this Si electrode-promoted flow cell, p+-Si/TiO2/Ni was used as dark electrode (Fig. S8). The current density of dark electrode continuously decreased during the 10 h stability test, having the same trend of decline as the Si photoelectrodes (Fig. S17). J–V curves of p+-Si/TiO2/Ni dark electrodes show no degradation during its 10 h stability test (Fig. S16). However, the formation of carbonate on the GDE surface (photograph in Fig. 15) was shown to inhibit CO2 transport and reduce the number of available catalytic sites after a 12 h stability test in dark (Fig. S17), leading to a decreased current density (Fig. 2c)47,48. Furthermore, carbonation of the electrolyte tends to shift the pH towards the neutral regime (from 13.7 to 13.4), resulting in higher solution resistance and unfavorable kinetics for OER, consistent with former investigations49,50. Therefore, the increased cell voltage can be attributed to the formation of carbonate on the GDE and the decreased pH of electrolyte.
Bae, D. et al. Back-illuminated Si-based photoanode with nickel cobalt oxide catalytic protection layer. ChemElectroChem 3, 1546–1552 (2016).
Ni was deposited by custom-made direct current magnetron sputtering. The sputtering target is Ni with high purity (99.99%). The chamber base pressure was kept at 10−5 Pa, and then high-purity Ar flow was fed into the chamber. The gas flow was controlled at 20 sccm by mass flow controllers. The process pressure was fixed at 1 Pa and deposition time can be regulated to adjust the thickness of the film.
The consequence of numerical aperture is that it directly relates to the Depth Of Field (DOF). For a given objective, looking at a sample, there’s a particular plane of perfect focus. The depth of field is, how far above and below that plane the objective and sample can be and still have everything in focus.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Sahli, F. et al. Fully textured monolithic perovskite/silicon tandem solar cells with 25.2% power conversion efficiency. Nat. Mater. 17, 820–826 (2018).
Zhou, X. et al. Solar-driven reduction of 1 atm of CO2 to formate at 10% energy-conversion efficiency by use of a TiO2-protected III-V tandem photoanode in conjunction with a bipolar membrane and a Pd/C cathode. ACS Energy Lett. 1, 764–770 (2016).
When it comes to digital imaging sensors, there is a wide variety to choose from. The main camera sensors used are CCD (charge-coupled device) or CMOS, Complementary Metal Oxide Semiconductor. For cutting edge performance, to reduce read noise, large and expensive cameras exist with liquid or Peltier coolers (deep cooled to about -60 degrees Celsius). Also, EMCCD (Electron Multiplied CCD) cameras allow for single photon detection and are popular for live cell imaging applications.
Van Deelen, T. W., Hernández Mejía, C. & de Jong, K. P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2, 955–970 (2019).
This paper describes the design and realization of a PEC CO2RR system that integrates a Si-based narrow bandgap photoelectrode to convert solar energy into chemicals at high photocurrent densities. The light absorption and catalytic sites could be separated onto different sides of the opaque Si photoelectrode, eliminating the tradeoff between optimized light absorption and efficient CO2 reduction in flow cells. The Si photoelectrode-assisted flow cell affords a high Faradaic efficiency of 90% and 53% for CO or C2+ products respectively, achieving the solar-to-fuel (STF) conversion efficiency of 2.42% for CO products. This work provides a promising design strategy for Si-based solar fuel production.
Liu, B. et al. Bifacial passivation of n-silicon metal-insulator-semiconductor photoelectrodes for efficient oxygen and hydrogen evolution reactions. Energy Environ. Sci. 13, 221–228 (2020).
The minority carrier diffusion length of Si photoelectrode can be calculated by Eq. (6), where τp is the minority carrier lifetime of n-type Si, Dp is the diffusion coefficient of hole (Dp = 12.4 cm2/s, at 298 K)55,56.
PEC measurements were performed using a three-electrode configuration with Si electrode as the working electrode, Hg/HgO as the reference electrode in alkaline electrolyte. Schematic illumination and photograph of the apparatus used in the PEC measurement are shown in Fig. S22. The potentials were converted into values against reversible hydrogen electrode using the Nernst Eq. (2) for dark cathode and photoanodes.
Zheng, J. et al. Efficiency and stability of narrow-gap semiconductor-based photoelectrodes. Energy Environ. Sci. 12, 2345–2374 (2019).
When designing automated digital microscopy devices for life science, biomedical, and diagnostic applications, typically, our goal is to optimize the device for the highest resolution image and the highest throughput in images per second. Microscope calculations such as magnification, resolution, microscope field of view, depth of field, and numerical aperture help us determine various aspects of a microscope’s capabilities and simplify the modeling and prototyping process. In this article, we will cover the importance of microscope calculations in optimizing microscope performance for your specific application.
Czochralski (CZ) c-Si wafers (n-type) with the resistivity of 1–5 Ω cm, 155 μm thickness and (100)-orientation were used as the substrate for Si electrode peparation. Silicon wafer was first textured using a mixture of potassium hydroxide (KOH) and isopropyl alcohol (IPA) (Top view SEM images in Fig. S1), then cleaned by standard RCA cleaning methods and finally dipped in 5% HF for 60 s to get rid of surface native oxide thin film. The Si wafer was then put into the PECVD chamber. 5 nm Intrinsic amorphous Si thin films were used to passivate the front and back side surfaces of Si substrate. n+ amorphous Si and p+ amorphous Si were deposited on the intrinsic amorphous Si (Fig. S4). WCT-120 lifetime tester from Sinton Instruments was applied for minority carrier lifetime test.
Ren, X. et al. Self-assembled CoOOH on TiO2 for enhanced photoelectrochemical water oxidation. J. Energy Chem. 60, 512–521 (2021).
Zhang, N., Long, R., Gao, C. & Xiong, Y. Recent progress on advanced design for photoelectrochemical reduction of CO2 to fuels. Sci. China Mater. 61, 771–805 (2018).
Silicon (Si), a narrow bandgap photoabsorber, is a promising photoanode candidate to drive the PEC CO2RR in flow cells due to its high saturation current density (larger than 40 mA/cm2)4,28,29,30,31. Moreover, single-junction Si solar cells are cheaper and have simpler fabrication processes than multi-junction solar cells designed for outer space applications18,32,33. However, most conventional Si photoanodes operate in the front-illumination mode (where illumination occurs on the same side as the catalysts and junction) because of its opaque nature, which makes it difficult to introduce light from its back side, and thus cannot be adapted to flow cell configurations34,35,36. Si with back-illuminated configurations is an elegant solution to this conundrum, as they decouple the location of light absorption and surface reaction sites37,38,39. Therefore, the implementation of back-illuminated Si photoelectrodes into PEC CO2RR flow cells has the potential to improve their energy utilization efficiency.
A larger field of view is generally desirable, as it allows for a larger area of the sample to be viewed at once, making it easier to locate and navigate to specific areas of interest. However, as the magnification increases, the field of view decreases, making it more difficult to observe larger areas of the specimen at higher magnifications.
Hegde, R. I., Hobbs, C. C., Dip, L., Schaeffer, J. & Tobin, P. J. Impact of metal-oxide gate dielectric on minority carrier lifetime in silicon. Appl. Phys. Lett. 80, 3850–3852 (2002).
Leonard, M. E., Clarke, L. E., Forner-Cuenca, A., Brown, S. M. & Brushett, F. R. Investigating electrode flooding in a flowing electrolyte, gas-fed carbon dioxide electrolyzer. ChemSusChem 13, 400–411 (2020).
Hu, S. et al. Amorphous TiO2 coatings stabilize Si, GaAs, and GaP photoanodes for efficient water oxidation. Science 344, 1005–1009 (2014).
Sony sensor
Kistler, T. A., Um, M. Y., Cooper, J. K., Sharp, I. D. & Agbo, P. Exploiting heat transfer to achieve efficient photoelectrochemical CO2 reduction under light concentration. Energy Environ. Sci. 15, 2061–2070 (2022).
Czochralski (CZ) p-type c-Si wafers with the resistivity of 0.001–0.005 Ω cm, 525 μm thickness and (100)-orientation were used as substrate. The ohmic back contacts were formed by rubbing the back side surfaces of the Si samples with a Ga–In alloy (99.9% metal basis, Alfa Aesar).
Where n is the number of electrons transferred to CO2 to produce a given product (n(CO) = 2; n(C2H4) = 12; n(C2H5OH) = 12), p0 is the pressure (101.325 kPa) and T = 273.15 K, R is the gas constant (8.314 J mol–1 K–1).
Liu, B. et al. Double‐side Si photoelectrode enabled by chemical passivation for photoelectrochemical hydrogen and oxygen evolution reactions. Adv. Funct. Mater. 31, 2007222 (2020).
Kistler, T. A., Um, M. Y., Cooper, J. K., Sharp, I. D. & Agbo, P. Monolithic photoelectrochemical CO2 reduction producing syngas at 10% efficiency. Adv. Energy Mater. 11, 202100070 (2021).
Chang, X., Wang, T. & Gong, J. CO2 photo-reduction: insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 9, 2177–2196 (2016).
During electrolysis, the gas mass flow controller was set to 20 sccm, the peristaltic pump was set to 30 rpm (photograph in Fig. S23), gas products were quantified using an online gas chromatography system (GC7890B, Agilent Technologies, Inc.). H2, O2, and N2 were detected by thermal conductivity detector (TCD) (MolSieve 5A packed column, Agilent Technologies, Inc.) and CO was detected by back-flame ionization detector (FID) (Porapak Q packed column, Agilent Technologies, Inc.). A methanizer was installed to enable the back FID to detect CO with 1000 times higher sensitivity. Ar was used as the carrier gas. After passing through the reactor, the gas was allowed to flow directly into the gas sampling loop of the gas chromatography for online gaseous product analysis.
Liu, L. X. et al. Highly efficient photoelectrochemical reduction of CO2 at low applied voltage using 3D Co-Pi/BiVO4/SnO2 nanosheet array photoanodes. ACS Appl. Mater. Interfaces 11, 26024–26031 (2019).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The same thing occurs in traditional photography, a very small aperture will increase the depth of field. A higher numerical aperture will give a higher resolution, but the depth of field becomes considerably smaller. There is a distance above the sample plane and a distance below the sample plane, and anywhere within them, there is essentially perfect focus. As soon as the objective and sample are outside of that boundary, the image begins to blur.
Kim, J. H. et al. Carbonate-coordinated cobalt co-catalyzed BiVO4/WO3 composite photoanode tailored for CO2 reduction to fuels. Nano Energy 15, 153–163 (2015).
Choi, S. K. et al. Sn-coupled p-Si nanowire arrays for solar formate production from CO2. Adv. Energy Mater. 4, 1301614 (2014).
One of the prerequisites for flow cell photoelectrode operation is for the photoelectrode to generate a significant photocurrent, allowing the reduction reaction to proceed at a high rate. Irtem et al. elegantly pioneered a TiO2 photoanode-assisted flow cell for the reduction of CO2 to HCOO−, which demonstrated the feasibility of employing photoelectrodes within flow cells22. In this configuration, however, only photoanodes with wide bandgaps such as TiO223,24, Fe2O325, WO326, BiVO427, etc. could only be used since light could be introduced from the back side of the photoanode through the FTO substrate, leaving the front side facing the cathode for reaction. The relatively small photocurrent density of wide bandgap photoanodes limits the reaction rate and changes the CO2 reduction product distribution. Therefore, photoelectrodes capable of generating high photocurrents are highly desired for PEC CO2RR flow cells.
The potentiostat (CompactStat.e20250, IVIUM) was used to measure the J–V curves and chronoamperometry (without iR compensation). J–V curves of samples were measured with a scan rate of 50 mV s−1 under the irradiation of a 150 W xenon lamp (SAN-EI ELECTRIC CO., LTD) equipped with an AM 1.5 G filter. The active geometric areas of the working electrode were calibrated by the software Image J.
Cheng, J., Zhang, M., Liu, J., Zhou, J. & Cen, K. A Cu foam cathode used as a Pt-RGO catalyst matrix to improve CO2 reduction in a photoelectrocatalytic cell with a TiO2 photoanode. J. Mater. Chem. A 3, 12947–12957 (2015).
The microscope field of view stands for the area of the sample visible through the microscope, which is calculated by dividing the sensor diagonal size by the magnification of the objective lens. For instance, a 20 mm diagonal sensor with a 20X objective lens would yield a Field Of View (FOV) of 1 mm, typical for microscopy applications. Microscope field of view can be calculated using the following formula:
The sample is throwing light out in all directions and the job of the objective is to collect as much of that light as possible. The way to do that is to have a high numerical aperture, a big wide cone. If the objective doesn’t collect a wide-angle of the cone, for example, a long working distance, low power objective will merely be getting the light that’s going straight through. That is why numerical aperture is the key to high-resolution imaging. There is one other variable that can be adjusted. By using bluer light, the resolution can be increased, but for a particular application, that may not be possible. Generally speaking, for any given objective, it is worth it to pay to get the highest possible numerical aperture, but there is a slight downside to that.
Joint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Binhai New City, Fuzhou, 350207, China
Sony IMX623 Datasheet
For 20X magnification, which can be a numerical aperture of 0.6 to 0.8, the depth of field drops to about plus or minus 500 nanometers. Moving into high magnification with oil immersion at a numerical aperture of 1.47, the depth of field drops very dramatically, And the depth of field could be plus or minus 0.1 to 0.2 microns (100 – 200 nm). That’s a very tight tolerance. At 100 nanometers or 200 nanometers, very tiny changes in flatness of the sample or the height of the sample, or the precision of the guideways of the XY sample motion stage, will make it tricky to stay in focus. For maintaining focus in this situation, a continuous tracking laser auto-focus system connected to a high bandwidth objective focusing stage, such as the DOF-5, is the ideal way to maintain focus in these high numerical aperture, high-resolution applications.
Arai, T., Sato, S., Kajino, T. & Morikawa, T. Solar CO2 reduction using H2O by a semiconductor/metal-complex hybrid photocatalyst: enhanced efficiency and demonstration of a wireless system using SrTiO3 photoanodes. Energy Environ. Sci. 6, 1274 (2013).
The applied bias photon-to-current efficiencies of photoanode were calculated using the J–V curves with an assumption of 100% Faradaic efficiency, according to Eq. (3). Where I (mA/cm2) is the photocurrent density under an applied bias of Vb (V vs. RHE), and P is the incident illumination intensity (mW/cm2) (100 mW/cm2 in this work).
To summarize, for high imaging resolution a high numerical aperture objective is required and one of the consequences of that is a fairly small depth of field which places an emphasis on the quality and performance of your focusing stage and XY sample positioning stage. Dover Motion has developed the DOF-5 specifically for microscope objective focusing.
Kim, B., Ma, S., Molly Jhong, H.-R. & Kenis, P. J. A. Influence of dilute feed and pH on electrochemical reduction of CO2 to CO on Ag in a continuous flow electrolyzer. Electrochim. Acta 166, 271–276 (2015).
For a low power 4x system, the numerical aperture is going to be very low, on the order of 0.05 to 0.1. In a medium power 40x system, it could be in the range of 0.5 to 0.8 and for a high power system, it can be as high as 0.9 or 0.95. As long as there is air between the objective and the sample, the numerical aperture can never exceed 1. When imaging slides, in order to exceed an NA of 1, a liquid can be added between the coverslip and the objective. Typically, oil or water are used for this and the objectives are referred to as oil immersion or water immersion objectives. With water, the numerical aperture goes up to about 1.1 and using oil, the numerical aperture can go up to as high as 1.47.
Murcia-Lopez, S. et al. Tailoring multilayered BiVO4 photoanodes by pulsed laser deposition for water splitting. ACS Appl. Mater. Interfaces 8, 4076–4085 (2016).
Xiong, L. et al. Breaking the linear scaling relationship by compositional and structural crafting of ternary Cu-Au/Ag nanoframes for electrocatalytic ethylene production. Angew. Chem. Int. Ed. 60, 2508–2518 (2021).
Lyu, Z. et al. Controlling the surface oxidation of Cu nanowires improves their catalytic selectivity and stability toward C2+ products in CO2 reduction. Angew. Chem. Int. Ed. 60, 1909–1915 (2021).
The surface recombination velocity can be calculated by Eq. (5) for the Si electrode with a-Si passivation, where W is the thickness of Si wafer, τbulk is the bulk minority carrier lifetime, S is the surface recombination velocity.
This is called the depth of field. The formula for the depth of field is: where: “n” is the refractive index of the material between the objective and the sample λ (lambda) is the wavelength of light NA is the Numerical Aperture
Kempler, P. A., Richter, M. H., Cheng, W.-H., Brunschwig, B. S. & Lewis, N. S. Si microwire-array photocathodes decorated with Cu allow CO2 reduction with minimal parasitic absorption of sunlight. ACS Energy Lett. 5, 2528–2534 (2020).
Sony STARVIS
J.L.G. supervised the project. J.L.G., T.W. and B.L. conceptualized the project. B.L., S.W., and G.Z. developed the concept and carried out the experiments and data analysis. Catalyst synthesis procedures were developed and performed by D.Z. and H.D. T.Y. and B.W. contributed to the development of photoelectrode fabrication method. All authors discussed the results and participated in writing of the manuscript.
All data generated or analyzed during this study are included in the published article and its Supplementary Information. Source data are provided with this paper.
Tang, P. Y. et al. Boosting photoelectrochemical water oxidation of hematite in acidic electrolytes by surface state modification. Adv. Energy Mater. 9, 1901836 (2019).
Sahara, G. et al. Photoelectrochemical reduction of CO2 coupled to water oxidation using a photocathode with a Ru(II)-Re(I) complex photocatalyst and a CoOx/TaON photoanode. J. Am. Chem. Soc. 138, 14152–14158 (2016).
Liu, B., Wang, T., Wang, S. et al. Back-illuminated photoelectrochemical flow cell for efficient CO2 reduction. Nat Commun 13, 7111 (2022). https://doi.org/10.1038/s41467-022-34926-x
Sensor format
Photoelectrochemical CO2 reduction reaction flow cells are promising devices to meet the requirements to produce solar fuels at the industrial scale. Photoelectrodes with wide bandgaps do not allow for efficient CO2 reduction at high current densities, while the integration of opaque photoelectrodes with narrow bandgaps in flow cell configurations still remains a challenge. This paper describes the design and fabrication of a back-illuminated Si photoanode promoted PEC flow cell for CO2 reduction reaction. The illumination area and catalytic sites of the Si photoelectrode are decoupled, owing to the effective passivation of defect states that allows for the long minority carrier diffusion length, that surpasses the thickness of the Si substrate. Hence, a solar-to-fuel conversion efficiency of CO of 2.42% and a Faradaic efficiency of 90% using Ag catalysts are achieved. For CO2 to C2+ products, the Faradaic efficiency of 53% and solar-to-fuel of 0.29% are achieved using Cu catalyst in flow cell.
The Faradaic efficiencies of the gas products were calculated by using the concentrations (ppm) detected by the GC as following Eq. (4):
The back-illuminated Si photoanode has been successfully coupled with the flow cell, and maintained a decent activity and stability for converting CO2 to CO, C2H4 and C2H5OH. The back-illuminated Si photoanode effectively reduced the energy consumed from the external electrical power supply. To assess the contribution of Si photoanode to the CO2 reduction system, the STF conversion efficiency of the Si promoted cell was calculated by Eq. (1):
In most cases the material between the objective and sample is air, and “n” equals 1.00. For water, it’s refractive index is 1.33, and specialized immersion oil for microscopy is 1.52. For a low magnification objective such as a 4X or even a 10X, the typical depth of field is plus or minus 3 to 5 microns. In that case, if the sample is very flat, focusing may not be required at all, or only one needs to happen one time. Generally speaking, samples vary in thickness and they vary in flatness, so focusing is required.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Li, C. et al. Photoelectrochemical CO2 reduction to adjustable syngas on grain-boundary-mediated a-Si/TiO2/Au photocathodes with low onset potentials. Energy Environ. Sci. 12, 923–928 (2019).
In this video, we will explain key optical imaging formulas and how they help in designing your automated digital microscopy imaging applications.
Ma, M., Trzesniewski, B. J., Xie, J. & Smith, W. A. Selective and efficient reduction of carbon dioxide to carbon monoxide on oxide-derived nanostructured silver electrocatalysts. Angew. Chem. Int. Ed. 55, 9748–9752 (2016).
Li, H. et al. Controllable distribution of oxygen vacancies in grain boundaries of p-Si/TiO2 heterojunction photocathodes for solar water splitting. Angew. Chem. Int. Ed. 60, 4034–4037 (2021).
Backsideilluminated
a STF efficiency of PEC Si photoanode for the conversion of CO2 to CO and b Performance comparison of photoanodes and photocathodes promoted cell for PEC CO2RR. Chart visualizing data on FE of products, photocurrent density, and STF for PEC CO2RR. Details are provided in Tables S1, S2.
In summary, this paper demonstrates a PEC flow cell for CO2 reduction with a back-illuminated Si photoanode that improves energy utilization efficiency. To overcome the contradiction between the opaque semiconductor light absorber and gas diffusion electrode in a flow cell, the Si photoelectrode was passivated by amorphous Si to obtain an ultra-long minority carrier diffusion length and separate the light absorption region from the catalytic sites. This decoupled structure enables the light to be introduced from the anode compartment side instead of the cathode side, when the photoanode is used as a sidewall of the cell. In this configuration, a high reaction rate (38 mA/cm2) for CO2 reduction was achieved in the PEC flow cell for CO2RR. For the conversion of CO2, the back-illuminated Si photoanode-assisted PEC flow cell shows an STF efficiency of 2.42% for CO with a FE of 90% using Ag catalyst. The direct PEC flow cell for CO2RR to C2+ products is achieved at a FE of 53% on Cu catalyst and an STF of 0.29% is achieved. The design strategy in this work opens promising approaches based on Si photoelectrodes to generate solar fuels from CO2 and H2O towards industrial application.
Liu, G. et al. Manipulating intermediates at the Au-TiO2 interface over InP nanopillar array for photoelectrochemical CO2 reduction. ACS Catal. 11, 11416–11428 (2021).
Li, H. et al. Construction of uniform buried pn junctions on pyramid Si photocathodes using a facile and safe spin-on method for photoelectrochemical water splitting. J. Mater. Chem. A 8, 224–230 (2020).
Zhang, Y., Luc, W., Hutchings, G. S. & Jiao, F. Photoelectrochemical carbon dioxide reduction using a nanoporous Ag cathode. ACS Appl. Mater. Interfaces 8, 24652–24658 (2016).
IMX811
Kim, J. H., Hansora, D., Sharma, P., Jang, J. W. & Lee, J. S. Toward practical solar hydrogen production—an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 48, 1908–1971 (2019).
LaTempa, T. J., Rani, S., Bao, N. & Grimes, C. A. Generation of fuel from CO2 saturated liquids using a p-Si nanowire || n-TiO2 nanotube array photoelectrochemical cell. Nanoscale 4, 2245–2250 (2012).
Dong, W. J. et al. Silver halide catalysts on GaN nanowires/Si heterojunction photocathodes for CO2 reduction to syngas at high current density. ACS Catal. 12, 2671–2680 (2022).
Wang, T. et al. Transparent Ta2O5 protective layer for stable silicon photocathode under full solar spectrum. Ind. Eng. Chem. Res. 58, 5510–5515 (2019).
We acknowledge the National Key R&D Program of China (2021YFA1500804), the National Natural Science Foundation of China (22121004, 22038009, and 51861125104), the Natural Science Foundation of Tianjin City (18JCJQJC47500), Haihe Laboratory of Sustainable Chemical Transformations (CYZC202107), the Program of Introducing Talents of Discipline to Universities (No. BP0618007) and the Xplorer Prize for financial support.
Song, Y. et al. B-Cu-Zn gas diffusion electrodes for CO2 electroreduction to C2+ products at high current densities. Angew. Chem. Int. Ed. 60, 9135–9141 (2021).
In addition to affecting the observation of the specimen itself, the field of view can also impact the ability to capture high-quality images and videos. A larger field of view requires a larger camera sensor or eyepiece, which can increase the cost of the microscope system. It is essential to consider the balance between field of view, magnification, and cost when selecting and optimizing a microscope for a particular application.
School of Chemical Engineering and Technology; Key Laboratory for Green Chemical Technology of Ministry of Education, Tianjin University, Tianjin, 300072, China
For the conversion of CO2 to CO, the FE of CO was larger than 90% in the voltage range of 0.3–1.334 V (Fig. 3b), leading to a high STF efficiency of 2.42% (Fig. 5a). To the best of our knowledge, this is the highest STF efficiency among photoanodes assisted cell for PEC CO2RR (Fig. 5b), also surpassing the STF efficiency of state-of-the-art photocathodes for PEC CO2RR (Fig. 5b). The STF for C2+ products reaches 0.29% (Fig. S21), which is lower than that of CO because the generation of C2H4 and C2H5OH (8 and 12 electrons reactions, respectively) is kinetically more difficult than the two-electron reduction products (CO)20,54. Although the STF efficiency in this work is lower than photoelectrodes fabricated from multi-junction III–V semiconductors under concentrated illuminations, the Si-based PEC flow cell holds the merit of low costs and facile fabrication processes18,32. Therefore, the Si photoanode could effectively reduce the high overpotential in OER, while maintaining a reasonable performance to produce CO2 reduction to CO or C2+ products compared with the conventional electrochemical CO2 reduction in the flow cell.
Chen, Z. et al. Grain-boundary-rich copper for efficient solar-driven electrochemical CO2 reduction to ethylene and ethanol. J. Am. Chem. Soc. 142, 6878–6883 (2020).
10 nm of amorphous TiO2 was deposited onto the above Si photoelectrodes at 150 °C in a custom-made ALD system, using Titanium (IV) isopropoxide (TTIP, Sigma-Aldrich, ≥99.9999%) and ultrapure water as precursors. The precursors were held at 70 °C and 25 °C, respectively. One ALD cycle consists of TTIP dose for 1 s, N2 purge for 10 s, water dose for 0.1 s and N2 purge for 10 s. The growth per cycle for TiO2 is 0.3 Å/cycle.
Wang, K. et al. Steering the pathway of plasmon-enhanced photoelectrochemical CO2 reduction by bridging Si and Au nanoparticles through a TiO2 interlayer. Small 18, e2201882 (2022).
Irtem, E. et al. A photoelectrochemical flow cell design for the efficient CO2 conversion to fuels. Electrochim. Acta 240, 225–230 (2017).
To construct a decoupled Si photoanode in which its illumination and catalytic sites are located on different sides and enable light absorption from the back side (Fig. 1b), amorphous Si was deposited as passivation layer to saturate the dangling bonds on Si surface due to its superior chemical passivation effect (prepared by plasma enhanced chemical vapor deposition (PECVD), details in Methods)28. The amorphous Si passivated the Si substrate, exhibiting an ultra-high minority carriers lifetime (3275 μs) (Fig. S3), and long minority carrier diffusion length of 2015 μm (calculation details in the Methods). This ultra-long minority carrier diffusion length is much longer than the thickness of Si wafer (155 μm), enabling photogenerated minority carriers (holes) to move from one side of Si electrode to other side before recombining (Fig. 1a). Thus, light absorption and surface reaction can be decoupled by having the light illumination side and OER catalyst layer on be located on different sides of Si substrate. Furthermore, two doped thin films (p type and n type amorphous Si) were deposited on amorphous Si (prepared by PECVD, details in Methods) to construct a built-in electric field to enhance the driving force for photogenerated carriers (Fig. S4).
a The schematic of the Si photoanode coupled flow cell with the gas diffusion electrode for PEC CO2RR. b The cross-section of back-illuminated Si photoanode and the GDE cathode (figure not to scale for clarity).
To realize a PEC flow cell for CO2RR, a Si photoelectrode integrated flow cell was proposed for CO2RR (Fig. 1a). The left sidewall of the flow cell is the Si compartment, where water oxidation reaction (OER) takes place. Incident light was irradiated from the chamber with the open-hole plate, allowing the Si to absorb the light directly. The surface of Si was textured to micro-pyramid (Fig. S1) to decrease the surface reflectance (Fig. S2). The GDE was situated on the opposite of the Si compartment, to facilitate CO2RR. Various catalysts can be deposited onto the GDE for different kinds of CO2 reduction products.
In the previous example we considered a sensor with 4 micron pixels used with an objective with 40X magnification and a numerical aperture of 0.8. The sensor and magnification provide 100 nm geometric resolution. However, due to diffraction, the sample image resolution will be greater than 100 nm. For example, yellow green light has a wavelength lambda of 550 nm. Using the above equation, the diffraction limited resolution is actually 419 nm. Therefore, the resolution of the sample is > 4 times the geometric resolution at the camera sensor which results in oversampling. While some oversampling is appropriate, 4 times is excessive and a waste of sensor pixels.
Duun, S., Haahr, R. G., Hansen, O., Birkelund, K. & Thomsen, E. V. High quantum efficiency annular backside silicon photodiodes for reflectance pulse oximetry in wearable wireless body sensors. J. Micromech. Microeng. 20, 075020 (2010).
a The schematic of Cu cathode. b The J–V curves of CO2RR with and without Si photoanode in a two-electrode configuration. c The FE of C2+ at different potentials. d Time-dependent photocurrents (left-axis) and FE toward C2+ (right-axis) under simulated AM 1.5 G illumination.
Besides the magnification, reducing the size of the sensor down to the size of the field of view on the sample also does the same with the pixels. For example, a sensor with 4 micron pixels and a 40X objective would be 0.1 micron of geometric resolution or 100 nanometers. But it turns out it isn’t quite that simple. There’s a property of light that acts like a particle, the photon. It also has a wave property. The wave nature of light leads to a condition called diffraction, and due to diffraction, limits are set on resolution. To better understand how that works, we need to explore another concept, which is called numerical aperture.
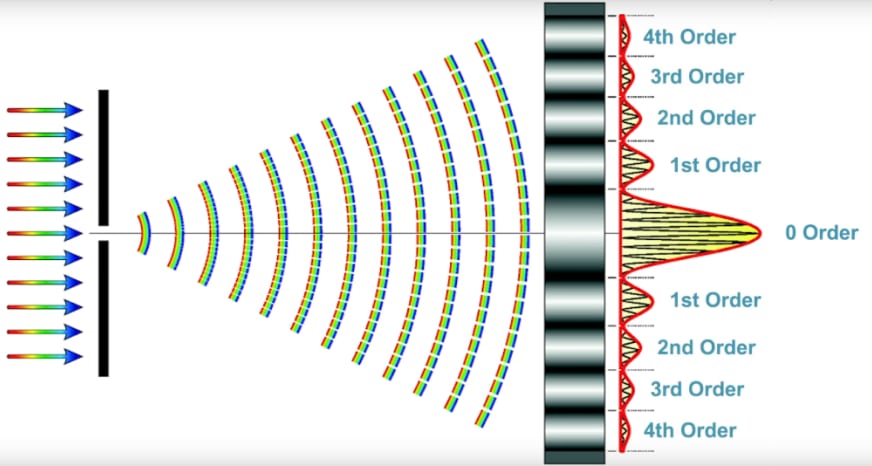
To better understand diffraction imagine if light moved strictly in straight lines. If a pinhole is illuminated with some light, the light would be directed in a straight beam. What actually happens is due to the wave nature of light, the light is diffracted, and instead of going straight, it spreads out into a cone. As shown in the diffraction image, the brightest light is the zero-order straight through, then the intensity decreases for the first order, second order, third order, etc. Unless the objective is capturing all of those higher orders, it is difficult to synthesize a high-resolution image. This describes what the fine structure of your sample is doing, it could be cells, chromosomes, or nuclei, and all of that fine structure spreads the light out.
To evaluate the performance and stability of back-illuminated Si photoanode for water oxidation. 10 nm TiO2 protective layer was deposited by atomic layer deposition (ALD). The thickness of TiO2 was optimized to 10 nm in our previous work40, as the thickness presents no significant effect on the stability of the silicon photoanode for water oxidation when the thickness exceeds 8 nm41. Then 5 nm Ni with a larger surface-active area compared to thick Ni thin films deposited by direct current (DC) sputtering on TiO2 as OER catalyst for Si photoanodes (photograph of Si photoanode in Fig. S5). The back-illuminated Si photoanodes exhibit a dense surface morphology after TiO2 and Ni deposition (Fig. S7). For PEC measurements, the Si photoanode was evaluated under AM 1.5 G illumination (100 mW/cm2). To be consistent, 1 M KOH (pH 13.7) was used as the CO2RR in flow cell electrolyte42. The Si photoanode showed an early onset potential of 0.8 V vs. reversible hydrogen electrode (RHE) and high saturation photocurrent density (38 mA/cm2) (Fig. 2a), a significantly reduced onset potential compared with electrochemical OER. This photocurrent density is also much higher than that of wide band-gap photoelectrodes (smaller than 5 mA/cm2), demonstrating the merits of narrow band-gap photoelectrode for CO2RR in flow cells. The photogenerated voltage of this back-illuminated Si photoanode, determined by the difference in onset potential between the Si photoanode and degenerated p+-Si/TiO2/Ni electrode with no photo response (Fig. 2a), was about 700 mV at current density comprised between 1 and 38 mA/cm2, as indicated. Holes were the majority and minority carriers of the p+-Si/TiO2/Ni dark electrodes, respectively. To fairly compare surface reactions between the Si dark electrode and photoelectrode, the TiO2 protective layer and Ni catalyst layer of dark electrodes were prepared under same batch as Si photoanode (Fig. S8). A high applied bias photon-to-current efficiency (ABPE) of 5.4% was obtained (Fig. 2b). The high ABPE and stability can be attributed to the efficient light utilization efficiency achieved by the back-illumination structure. Furthermore, the stability of Si photoanodes was evaluated by a chronoamperometry test at a fixed applied potential of 1.5 V vs. RHE. The photocurrent remains stable for over 150 h (Fig. 2c). The Faradaic efficiency of O2 generation for Si photoanode is higher than 97% at 1.5 V vs. RHE under AM 1.5 G irradiation over a 150-h, indicating that most of the photogenerated holes are consumed for OER (Fig. 2c). The surface morphology is nearly unchanged after the stability test (Fig. S7). Moreover, grazing incidence X-ray diffraction (GIXRD) patterns of Si photoanode showed similar ITO diffraction peak intensity before and after stability (Fig. S9). Ni catalyst layer was stabilized on the surface of TiO2 by metal–support interaction43, and partially transformed to NiOx or NiOOH after stability test (Fig. S6). This robust stability is attributed to the ALD-deposited TiO2 separating the electrolyte and Si photoanode, preventing corrosion. The superior activity and stability of this back-illuminated Si photoanode configuration have the potential to operate in flow cells with reduced cell voltage and improved energy utilization efficiency for CO2RR.
Thevenon, A., Rosas-Hernández, A., Fontani Herreros, A. M., Agapie, T. & Peters, J. C. Dramatic HER suppression on Ag electrodes via molecular films for highly selective CO2 to CO reduction. ACS Catal. 11, 4530–4537 (2021).
where Sensor Diagonal is the diagonal size of the camera sensor in millimeters (similar to specifying a TV size), and Objective Magnification is the magnification of the objective lens being used.
Li, A. et al. Three-phase photocatalysis for the enhanced selectivity and activity of CO2 reduction on a hydrophobic surface. Angew. Chem. Int. Ed. 58, 14549–14555 (2019).
In addition to CO, multi-carbon products with high energy densities such as C2H4 and C2H5OH are also producible from this PEC flow cell configuration20,51. 100 nm Cu was sputtered on carbon paper GDE in this work (photograph in Fig. S10b). X-ray diffraction and X-ray photoelectron spectroscopy confirmed the existence of metallic Cu (Figs. S18, S19). The Cu catalyst was combined with the back-illuminated Si photoanode to carry out the CO2RR in flow cell using 1 M KOH (pH 13.7) as the electrolyte (Fig. 4a) (photograph in Fig. S20).
Fàbrega, C. et al. Tuning the fermi level and the kinetics of surface states of TiO2 nanorods by means of ammonia treatments. J. Phys. Chem. C. 117, 20517–20524 (2013).
Microscope calculations are specific to the imaging sensor and microscope objective selection, which also impacts the performance requirements of the sample XY motion and Z focusing motion.
Fan, R. et al. Unassisted solar water splitting with 9.8% efficiency and over 100 h stability based on Si solar cells and photoelectrodes catalyzed by bifunctional Ni-Mo/Ni. J. Mater. Chem. A 7, 2200–2209 (2019).




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500