M12 4-pin A-code pin assignment : r/PLC - m12 color code
Several studies show epigenetic chromatin modulation by H2S [114, 115]. Slow-releasing H2S donors inhibit tumor growth both in vitro and in vivo by a combination of cell-cycle arrest and apoptosis promotion, which was related to histone hyperacetylation [116–118]. The transcription of pro-inflammatory cytokines, like IL-6 and TNF-α, was altered due to histone modifications and accompanying changes in chromatin structure [114]. Expression and activity of SIRT1 is mediated by H2S [10, 119, 120]. The protective effects of H2S against senescence [119, 121], apoptosis [90] and neurotoxicity of formaldehyde [120] were found to be modulated through SIRT1 activity and were attenuated by SIRT1 inhibition [90, 120]. Direct anti-apoptosis and antioxidant effects of H2S via the SIRT1 pathway were demonstrated in cells under oxidative stress [90, 122].
Find all translations of optica in English like optics and many others ... swap_horiz English English Definition; English ... What is the translation of "optica ...
Telomere attrition [232], DNA lesions [233], oxidative stress [234] and activated oncogenes [235, 236] are examples of stressors shown to induce senescence by the activation of the DNA damage response, which arrests cell-cycle through stabilization of p53 and transcriptional activation of p21 [226]. Other pathways, such as the p16INK4a/Rb and the p19ARF/p53 pathways, can also induce senescence, independent of the DNA damage response [226, 237]. These pathways were genetically linked to the highest number of age-related diseases [238] and correlated with the chronological age of essentially all tissues analyzed in both mice and humans [239, 240]. Other studies underscored the close relationship between metabolic changes and senescence [235, 241–243].
In experimental settings, levels of H2S can be manipulated by the administration of exogenous H2S or H2S releasing compounds. Sulfide-sodium salts, exposure to gaseous H2S, slow-releasing H2S donors, hybrids of H2S-donors and known substances, thiosulfate, cysteine analogs and modulation of the expression or activity of H2S producing enzymes are several options for altering H2S levels in experimental settings.
Digital single-lens reflex camera ... A digital single-lens reflex camera (digital SLR or DSLR) is a digital camera that combines the optics and mechanisms of a ...
Aging is the progressive loss of physiological function which emerges when organisms grow older, with death of the organism as the ultimate, inevitable consequence [1, 3, 11]. Classic symptoms of human aging include graying and loss of hair, loss of hearing and eyesight, reduced fertility, immune system failure and loss of cognition [2]. These classical symptoms decrease the quality of life of most elderly, but are generally accepted as inevitable consequences of aging. These symptoms are believed to result from time-dependent accumulations of cellular damage leading to a gradual loss of function at the molecular, cellular, tissue and organismal level [12, 13]. A variety of molecular, biochemical and metabolic alterations occurring at the cellular level are thought to cause these functional losses [2]. For many years, aging research was largely focused on identifying the underlying cellular mechanisms as to find novel drug targets to modulate the aging process and to attain healthy aging by delaying the onset of age-related pathologies [3]. This approach has led to some unsubstantiated and opposing claims for potential cures as opposed to disease-oriented research [14]. It turned out that the aging processes are highly complex with numerous mechanisms and pathways involved. Several theories and mechanisms explaining the biology of aging have been proposed [2]. These theories are not mutually exclusive and attempts to unify them into one theory have not yet succeeded. Therefore, the molecular and cellular pathways generally considered to contribute to the process of aging were categorized and proposed as the ‘hallmarks of aging’ [15].
The genomic stability systems also include specific mechanisms for maintaining the appropriate length and functionality of telomeres [91]. Telomere maintenance is, however, considered as a distinct hallmark of aging. The unique DNA-protein structure of telomeres is essential for maintaining genomic integrity, as they cap the terminal ends of linear chromosomes in order to prevent appearing as DNA double-strand breaks in need of repair and to protect these ends from degradation [92]. Normal aging in mammals is accompanied by the progressive and cumulative loss of telomere length and function as a consequence of normal replication, due to oxidative damage or as a result of replication errors [15, 92, 93]. Telomerase, a specialized DNA polymerase, is required to elongate telomeres as the replicative DNA polymerase lacks this capacity. However, most mammalian somatic cells do not express telomerase, making telomeres particularly susceptible to age-related deteriorations [91]. When telomeres become too short or when too much damage has occurred, they become dysfunctional. Dysfunctional telomeres are highly efficient in inducing apoptosis and/or cellular senescence and accelerate aging in mice and humans [15, 94, 95]. Experimental stimulation of telomerase was shown to delay or even reverse aging [15, 96].
ConnectorPins Female
Nowadays, H2S is recognized as an important signaling molecule with various (patho-)physiological effects and was shown to be involved in cardiovascular system diseases, cancer and neurodegenerative diseases [8, 10]. The most widely studied functions of endogenous H2S relate to its vasodilatory effects [23, 59, 60] and its ability to reduce and modulate oxidative stress. Additionally, several other physiological function of H2S signaling were proposed [10]. These studies demonstrated that H2S has the potential to provide beneficial effects on health in many (patho-)physio-logical processes and age-related pathologies. However, the effects of H2S on aging are not that commonly studied. Nevertheless, pathways of most – if not all – hallmarks of aging are found to be influenced by H2S. Studies that identified the effects of H2S on these pathways are thus relevant in the light of aging and are described next.
Stem cells regularly replace damaged or missing cells in tissues in order to maintain tissue homeostasis [249]. Most stem cells remain in their quiescent state and rarely enter cell-cycle in order to prevent the accumulation of damage during normal metabolic respiration and cell division [250]. However, not all damage can be prevented, resulting in a decline in stem cell function with age. As a consequence, their regenerative potential may be adversely affected, leading to organ failure and diseases of aging [250]. The previously discussed hallmarks are involved in the decline of stem cell function with age, as for example the accumulation of DNA damage [251] and telomere shortening [252, 253] were shown to attribute to stem cell exhaustion. In addition, stem cells are extremely sensitive to the loss of telomerase, which they normally express in order to maintain their genomic integrity [254, 255]. Stem cell attrition is thought not to be a direct result of the DNA damage itself but to be a product of the cellular responses to the damage, such as the activation of p53 that initiate DNA repair mechanisms and cell death programs [256, 257]. Studies also showed that stem cell function could be improved after calorie restriction [258, 259] or mTOR inhibition [259–261] in several tissues, resulting in improved proteostasis and affecting the energy sensing networks. A study in hematopoietic stem cells showed that epigenetic dysregulation during aging declined the regenerative potential and function of stem cells [262].
MultiPin ConnectorPlugs
H2S is also associated with altered DNA methylation. High plasma levels of homocysteine inhibit the CSE/H2S signaling and trigger mitochondrial toxicity, endothelial dysfunction and inflammation by increasing DNA methylation and transcriptional alterations, like elevation of TNF-α and IL-1β plasma levels and inhibition of CSE expression [123, 124]. Supply of H2S-releasing compounds rescued these cells from the harmful effects of high levels of circulatory homocysteine [123, 124]. Hydrogen sulfide alters the epigenetics of the mitochondrial genome, contributing to the replication of mitochondrial DNA, cellular energy metabolism and mitochondrial bioenergetics [125]. Exogenous H2S repressed the expression of DNA methyltransferase in cultured smooth muscle cells and aortic tissues from mice, resulting in the demethylation of certain regions of the mitochondrial genome, whereas CSE deficiencies showed opposite effects [125]. These studies demonstrate that epigenetics can be changed by alterations in the transsulfuration pathway.
Mitochondrial dysfunction and oxidative stress are thought to play an important role in aging by affecting intracellular signaling and interorganellar crosstalk [194–196]. Several age-related pathologies, like neurodegenerative diseases, diabetes, cancer, cellular senescence and impaired stem cell homeostasis were linked to ROS and dysfunctional mitochondria [197, 198]. Mild mitochondrial toxins, like metformin and resveratrol, retarded aging by inducing a low-energy state with increased AMP levels and AMPK activation [199] and by mediating the master antioxidant regulator Nrf2 [200]. These studies indicate that mild mito-chondrial stresses might be preventive against age-associated pathologies.
Several stem cell populations are affected by H2S. Endogenous H2S signaling maintains several biological functions of human periodontal ligament stem cells and neural stem cells, like the capacity to proliferate and differentiate [263–265]. Exogenous H2S may protect against neuronal decline normally observed after hypoxia [265]. In addition, NaHS increased and CSE inhibition decreased proliferation of human adipose tissue derived stem cells [266]. Bone marrow mesenchymal stem cells produce H2S in order to regulate their self-renewal and differentiation capacity [267]. Deficiencies in H2S signaling impaired stem cell function and bone homeostasis, which could be restored by H2S application [267]. Mesenchymal stem cells exposed to hypoxic conditions significantly decreased their H2S production and underwent apoptosis, whereas CSE overexpression protected against apoptosis [268]. Modulation of H2S signaling was suggested to be a potential therapeutic approach by which the viability of transplanted stem cells and the efficiency of cell-based therapy could be promoted [266, 268, 269].
Several other key chromosomal protein and chromatin remodeling factors are diminished in normal and pathological aged cells [111, 112]. Alterations in their expression altered the lifespan of flies [113], demonstrating their role in the aging process.
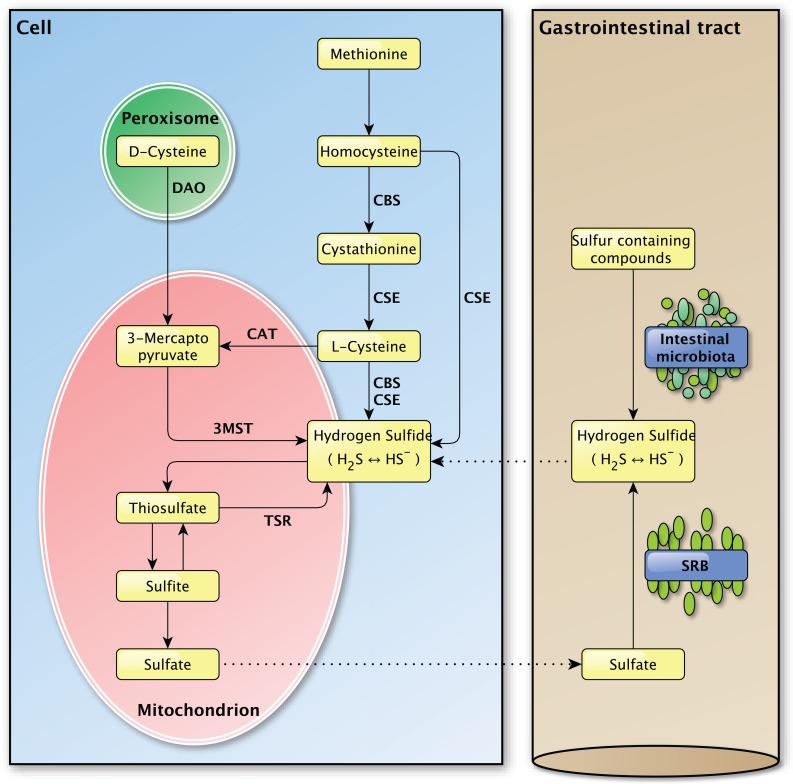
Left panel: The endogenous production of H2S in mammalian cells. Several important enzymes are mentioned along the arrows. Right panel: The exogenous production of H2S in the gastrointestinal tract by the intestinal microbiota and sulfate-reducing bacteria, for which the H2S production is endogenous. The dashed lines between the left and the right panel indicate the transport of molecules between the compartments.
ConnectorPins Male
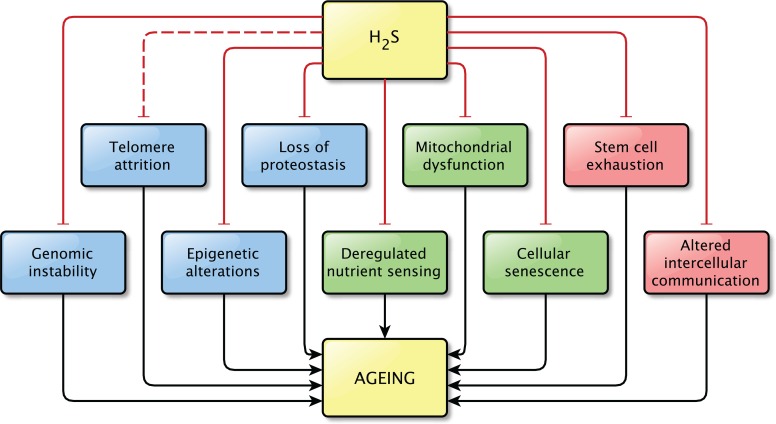
The precise relationship between H2S and aging still remains unknown. However, the findings discussed in this review strongly support the idea that H2S plays a role in the process of aging and in age-related pathologies, as direct effects on pathways related to aging were shown in all but one hallmarks of aging (Figure 3). It is possible that not all effects described here are important in the light of aging, but together they indicate that the contribution of H2S signaling to normal physiology and to pathophysiology is not to be neglected.
The mTOR pathway is another major nutrient-sensing pathway with effects on aging [159]. Animal studies showed that genetic downregulation of mTOR or treatment with the mTOR inhibitor rapamycin extended longevity in yeast, worms, flies and mice [160, 161]. Rapamycin also improved and protected against age-related pathologies in mouse models of Alzheimer's disease, Parkinson's disease and cardiomyopathy [162, 163]. Along with these beneficial effects, several harming side-effects of mTOR inhibition, like impaired wound healing, insulin resistance and cataract, were shown in mice [161, 164].
SYLVANIA LED light bars are put through rigorous quality testing to ensure premium quality and come with a Lifetime Limited Warranty.
Sonance Professional Series has become a game-changer for commercial audio, delivering clean aesthetics, excellent sonic performance, and consistent tonality.
After the conversion of methionine to homocysteine, CBS is required to form cystathionine from homocysteine whereupon CSE converts cystathionine to L-cysteine, the latter being the key substrate in the generation of H2S. Hydrogen sulfide can also be generated directly from homocysteine by CSE, but not by CBS [42]. L-cysteine is used by CBS and CSE to form H2S and by cysteine aminotransferase (CAT) to produce 3-mercaptopyruvate, the main substrate for H2S production by 3MST. In the presence of D-amino acid oxidase (DAO), 3-mercaptopyruvate can also be produced from D-cysteine in peroxisomes [43]. The presence of a dithiol is required for 3MST in order to release H2S [44]. In vivo, H2S is metabolized by methylation in the cytosol [16] or by oxidation in mitochondria, whereupon it is secreted as sulfite, thiosulfate and sulfate [36]. In vivo, hydrogen sulfide can be re-formed from sulfite and thiosulfate, but not sulfate [39, 45].
14.4 millimeters are equal to 0.0144 meters. More about meters and millimeters, the units used in the conversion under consideration, can be found in our ...
Sulfur-containing amino acids, like methionine and cysteine, are the main precursors for the enzymatic generation of H2S. CBS and CSE account for the majority of the endogenous produced H2S in mammalian tissues [16]. Their expression is tissue specific and was identified in liver, kidney and brain cells, skin fibroblasts and blood lymphocytes among others [16].
As discussed previously, H2S protects the mitochondrial genome from damage and thereby preserves the mitochondrial integrity and the cellular energetics [38, 89, 125, 222]. Together these actions induced by H2S protect the integrity of mitochondria and prevent mitochondrial dysfunction.
The effects of H2S on mitochondria are well described and its ability to reduce and modulate oxidative stress is considered to be one of the principal features of H2S in physiology. Several studies showed protective effects of H2S on mitochondrial function, as it increased the levels of antioxidants [213, 214], reduced the production of mitochondrial superoxides [215] and activated ROS-scavengers [216] and the anti-oxidative transcription factor Nrf2 [217, 218]. NaHS treatment stimulates the activities of superoxide dismutase and glutathione peroxidase and upregulated the expression of other antioxidants in human neuroblastoma cell line SH-SY5Y [143]. H2S improves mitochondrial ATP production in smooth muscle cells with impaired ATP production, especially following hypoxia [38]. Under stress conditions, cells can use H2S as an inorganic energy substrate for the mitochondrial respiratory chain to sustain ATP production [219–221]. It was proposed that high concentrations of H2S inhibit mitochondrial activity and protected organs against ischemia/reperfusion injury by reversible binding to cytochrome c oxidase, leading to hypometabolism, hypothermia and tissue preservation [79, 222–224].
H2S also regulates the activity of the low-energy sensing sirtuins. Treatment with NaHS enhanced the SIRT1 deacetylase activity in human umbilical vein endothelial cells [119, 121]. In addition, increases in the expression of SIRT1 [119, 120], SIRT3 [180, 192] and SIRT6 [180, 193] were shown after treatment with H2S.
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Nowadays, H2S is acknowledged as an endogenously produced signaling molecule with various (patho-) physiological effects. H2S is involved in several diseases including pathologies related to aging. In this review, the known, assumed and hypothetical effects of hydrogen sulfide on the aging process will be discussed by reviewing its actions on the hallmarks of aging and on several age-related pathologies.
202097 — You can cut a shower curtain or something similar to fit it. There's really limitless ways to diffuse light cheaply. Head down to a dollar store and peak ...
When humans grow older, they experience inevitable and progressive loss of physiological function, ultimately leading to death. Research on aging largely focuses on the identification of mechanisms involved in the aging process. Several proposed aging theories were recently combined as the ‘hallmarks of aging’. These hallmarks describe (patho-)physiological processes that together, when disrupted, determine the aging phenotype. Sustaining evidence shows a potential role for hydrogen sulfide (H2S) in the regulation of aging.
Electricalconnectorpins automotive
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Nowadays, hydrogen sulfide (H2S) is acknowledged as the third gasotransmitter [8, 16]. The biological and medical importance of the gasotransmitters NO, CO and H2S is now widely recognized. Whereas the endogenous concentrations of each gasotransmitter in the circulation or in tissues are relatively low, these concentrations are sufficient to execute their specific physiological actions [17–19]. Recently, other small gaseous molecules were evaluated for their candidacies as gasotransmitter and as ammonia (NH3) did meet all criteria for gasotransmitters it should be classified as the fourth gasotransmitter [17]. Over the past decade, evidence demonstrating the importance of gasotransmitters to the human body mounted as their regulatory capacities in controlling important physiological functions, like vascular tone, defense against pathogens, neuro-modulation, apoptosis and energy metabolism, were shown [20].
EMU is an easy-to-use audio to DMX software package that enables you to pre-program your entire light show in advance with it's advanced attribute selector and ...
Many studies demonstrated the importance of a proper protein homeostasis or ‘proteostasis’ in health and disease. Permanent or temporal alterations in proteostasis, as a result of changes in physiology or exposure to environmental stresses, are a common feature of development and cellular and organismal aging [126, 127]. Several age-related pathologies, like Alzheimer's and Parkinson's disease, are characterized by the appearance of misfolded or aggregated proteins with disease-causing, proteotoxic effects [128, 129]. In order to preserve the integrity, stability and functionality of the proteome, all cells take advantage of a network of quality control pathways that coordinate the synthesis, folding, disaggregation and degradation of proteins and their posttranslational modifications [15, 127]. This proteostasis network includes the translational machinery, molecular chaperones, the ubiquitin-proteasome system and the lysosomal autophagy machinery [129]. Several signaling pathways were shown to assist and modify the proteostasis network but are not considered as direct components of that network [127]. Molecular chaperones, like proteins of the heat-shock family, mediate the folding and stabilization of correctly folded proteins [126, 130]. Studies showed the importance of chaperones in the regulation of lifespan in several model organisms [131–133]. However, the efficacy of protein quality control by these chaperones declines with aging [134]. Affected proteins are normally targeted to destruction by the ubiquitin-proteasome or the lysosomal autophagic pathways [126, 135]. However, the activities of these proteolytic systems also decline with aging [136, 137]. Numerous studies showed that the induction of these pathways extended longevity in several animal models [136, 138–141]. Together, these studies support the idea that collapsing proteostasis contributes to the aging process.
Administration of L-cysteine or Na2S to human U937 monocytes exposed to high-glucose, as a model for diabetes, increased cellular PIP3, AMPK phosphoryla-tion and PPARγ expression [181]. CSE inhibition prevented the L-cysteine-induced increase in PIP3 [181]. Various H2S-releasing compounds activated AMPK signaling, resulting in protective effects on cultured cells, tissues and whole organisms [182–186]. Interestingly, the well-established AMPK activator metformin, a widely prescribed insulin sensitizer and a first-line antidiabetic drug, increased H2S concentration in various tissues [187], indicating that H2S might mediate metformin's effects. H2S was shown to promote AMPK signaling and inhibit mTOR complex 1 activity in renal epithelial cells [188]. NaHS treatment blocked mTOR activity in Werner syndrome fibroblasts [147] and the human colon adenocarcinoma cell line HR-29 [189]. Contrary, NaHS treatment increased mTOR phosphorylation in reperfused hearts and concanavalin A-induced hepatitis [190, 191].
Historically, hydrogen sulfide was known as a toxic gas characterized by the strong odor of rotten eggs. Until the 1990s, the research on H2S was mainly focused on its toxicity. Accidents in industrial settings showed the danger of the gas, as exposure to high concentrations of H2S caused collapse, unconsciousness and respiratory paralysis, and ultimately led to death [25]. Since then, new knowledge has positively transformed the way H2S is perceived, as studies discovered physiological functions of H2S in biological systems [26, 27] and showed endogenous production of H2S in many parts of the mammalian body [8, 28]. As H2S is soluble in both water and lipids, it easily penetrates biological membranes without facilitation of membrane channels [29]. The term ‘H2S’ in this review refers to H2S and its anions, as more than 80% of this weak acid dissociates to hydrosulfide anion (HS−) and proton (H+) at the physiological pH (pH=7.4) in the circulation and the cells [30]. The contribution of the other sulfide anion (S2−) is negligible at physiological conditions, as the dissociation of HS− to S2− occurs almost exclusively at high pH [29, 30]. It is not possible to separate the effects of H2S and HS− on physiological functions and signaling processes since these species coexist at physiological conditions in cells and in circulation [29, 31, 32].
Along with the genetic components in aging, a role for non-genetic factors in the aging process is implicated [97]. Epigenetic alterations such as posttranslational modification of histones, alterations in DNA methylation patterns and chromatin remodeling [15, 97–99], can regulate the accessibility of the DNA and underlie the differential gene transcription observed between cell types, developmental stages and disease states [100–102]. Several histone modifications are associated with aging [103, 104], such as the deacetylase activity of sirtuins. Manipulation of a single sirtuin gene in several animal models resulted in noteworthy effects on longevity by slowing down organismal aging [105]. At least three members of the sirtuin family, namely SIRT1, SIRT3 and SIRT6, are involved in the regulation of longevity and/or healthy aging in mammals [15]. The underlying mechanisms by which they affect these processes differ between the sirtuins [15].
Several lines of evidence indicate the involvement of H2S in cellular and organismal senescence. NaHS protects human umbilical vein endothelial cells against cellular senescence, potentially through the modulation of SIRT1 activity, and improves the function of senescent cells [119, 121]. Cellular aging can be delayed by decreasing oxidative stress. H2S protects against stress-induced cellular senescence by initiating the antioxidant responses, for example by S-sulfhydration of Keap1 leading to the activation of Nrf2 [218] and by inhibition of mitochondrial ROS production, by S-sulfhydration of p66Shc which prevents its phosphorylation and activation [247]. Others demonstrated that H2S induced the S-sulfhydration of MEK1, leading to PARP-1 activation and DNA damage repair, protecting cells from senescence [86]. Caloric restriction, reported to decelerate the biological aging process, maintained normal H2S levels, reduced oxidative stress-induced cellular senescence and promoted cellular cyto-protective systems [248]. In addition, deficiency of CSE in mouse embryonic fibroblasts led to early development of cellular senescence [218], under-scoring the regulative role of H2S signaling in senescence.
The previously discussed sirtuins are part of the nutrient signaling pathway, as these NAD-dependent protein deacetylases respond to cellular low-energy states, cause epigenetic alterations and contribute to the aging process [15, 170].
Hydrogen sulfide is endogenously produced by most mammalian cells via enzymatic and non-enzymatic pathways [8, 28]. However, the contribution of the non-enzymatic pathway, in which elemental sulfur or organic polysulfides are reduced to H2S, is small [16, 33, 34]. Stressful conditions, like increased oxidative stress and hyperglycemia, promote H2S production from this pathway [8].
Besides the destabilizing effects of alterations in the nuclear and mitochondrial genome, the stability of the genome can also be deteriorated by defects in the nuclear architecture [67]. Alterations in the nuclear lamina have been detected in disease and during normal aging [68, 69], causing changes in several stress pathways [70, 71] and attrition of adult stem cells [72, 73]. The lifespan of progeroid mice was extended after these pathways were restored [71, 74], indicating their importance in the aging process.
Exogenous and endogenous treats continuously challenge genome integrity and stability [61]. Organisms have evolved a complex network of repair, damage tolerance and checkpoint mechanisms to counteract most damage caused to the nuclear genome [15, 61, 62]. Artificial reinforcements in these mechanisms extended healthy lifespan in mice [63] and were suggested to delay aging [15]. In contrast to the nuclear genome, the protection of mitochondrial DNA is not that efficient [64] and is heavily dependent on the machinery of nuclear DNA repair [65]. Besides that, mitochondrial DNA is more vulnerable to mutations due to the oxidative microenvironment of the mitochondria and the lack of protective histones on mitochondrial DNA [64]. Therefore, aging-associated mutations and deletions in the mitochondrial genome may also contribute to the aging process [66].
... syringes and needles. Use The 18G diameter of the needles is suitable for sampling ... Deutsch Deutsch. Start typing and press Enter to search. Judge.me.
In the premature aging disorder Werner syndrome, fibroblasts are highly stressed with extensive protein production and aggregation in the cytosol accompanied by nuclear dysmorphia [147]. Treatment with NaHS or rapamycin normalized the morphological phenotype and restored proteostasis by blocking mTOR activity and annulling protein aggregations [147]. These studies demonstrate that H2S can influence the maintenance of cellular protein homeostasis.
Pin ConnectorSocket
No studies have specifically described the effect of H2S on telomere maintenance, but the described protective effects of physiological levels of H2S on genome stability might also preserve telomeres and prevent telomere attrition and dysfunction.
Besides histone modification, DNA methylation also alters chromatin structure and regulates gene expression [106]. Alterations in DNA methylation are considered to be a central mechanism in normal development, some diseases and in the aging process [107] and accumulate during life, as was shown in a study in centenarians and newborns [108]. However, the relationship between DNA methylation and aging is complex, as both a global decrease in DNA methylation, as well as specific age-related hypermethylation at certain developmentally regulated genes, including various tumor suppressor genes and Polycomb target genes, were described in different human tissues during aging [15, 98, 108, 109]. Among the differentially methylated regions, some were shown to be related with age or were associated with age-related phenotypes [110]. Nevertheless, direct experimental evidence showing that organismal lifespan can be extended by altered DNA methylation patterns was not found thus far.
Aside from endogenous H2S production, H2S can be produced by bacteria in the mouth and the gastrointestinal tract (Figure 2). From the perspective of the host-organism, this H2S production is exogenous as it is produced by bacteria in the external milieu of the host and not by that organism itself. Sulfur-reducing bacteria, for example, are part of the normal intestinal microbiota in healthy individuals and have been identified in human feces [46]. Hydrogen sulfide is synthesized by these bacteria from alimentary as a consequence of their metabolic activity in which they use sulfate as a terminal electron acceptor [8, 47]. The main source of this inorganic sulfate is the aliment, however the secretion of sulfate by the host gastrointestinal tract may also be an important source [48]. As the intestine is highly permeable to H2S [49], the production of high concentrations of H2S by these bacteria would be expected to cause severe tissue damage [50]. The exogenous production of H2S was also suggested to play a role in several pathologies of the intestinal tract, like inflammatory bowel disease and colorectal cancer, but direct links are yet to be established [51–53]. The intestinal mucosa is thought to protect the gastrointestinal tract against high concentrations of H2S by a specialized detoxification system which rapidly and effectively metabolizes H2S to thiosulfate and sulfate [8, 50], whereupon sulfate can be secreted [48]. It is possible that defects in this detoxification pathway play a part in intestinal pathologies [49, 50]. The importance of proper H2S catabolism is demonstrated in patients with ethyl-malonic encephalopathy, a disease in which malfunction of the persulfide dioxygenase ETHE1 results in multi-organ pathology in the brain, gastrointestinal tract and peripheral vessels for example [54]. Due to mutations in the ETHE1 gene [55], the inorganic sulfur catabolism is impaired which leads to the accumulation of H2S and various H2S-related toxic effects in different tissues [54, 56, 57]. Decreased exogenous H2S production, due to treatment with the antibiotic metronidazole, and improved H2S buffering, by administration of the glutathione precursor N-acetylcysteine, caused marked clinical improvements in patients with ethylmalonic encephalopathy [58]. The exact role of exogenously produced H2S in (patho-) physiology still needs to be determined and separated from the effects of endogenously produced H2S [53].
AMP-activated kinase (AMPK) is a highly conserved nutrient and low-energy sensor [15, 165, 166]. Besides its functions in the maintenance of energy metabolism, it was suggested that AMPK coordinates a large signaling network of transcription factors [165, 167], including pathways involved in aging [166]. Several studies on model organisms demonstrated the crucial role of AMPK in the regulation of longevity in worms, flies and mice [166]. The responsiveness to AMPK signaling declines with age [168], contributing to many age-associated diseases [169].
Over the past decades the worldwide human life expectancy has increased substantially [1]. This, combined with several other factors, led to a dramatic and ongoing increase in the proportion of the world's human population aged over 60 years [2]. As a consequence, the number of people experiencing age-related deterioration in health is rising, which will cause a great burden on the healthcare system [2]. The deterioration of the physiological integrity leads to an increased incidence of major human pathologies and increases the probability of death [1, 3]. Age remains the main risk factor for the development of debilitating and life-threatening, age-related pathologies such as cardiovascular disease, cancer, type 2 diabetes mellitus and neurodegenerative disorders including dementia [1–4]. Understanding what causes aging and how aging and age-related diseases are interrelated is therefore essential. This led to an increased interest in aging research and made healthy aging a hot topic in research.
Contributions of H2S signaling to several nutrient sensing pathways are described. H2S impairs the insulin/IGF-1 signaling pathway as it inhibits insulin secretion by pancreatic beta-cells and insulin secreting cell lines by stimulating ATP-sensitive potassium channels [171–173]. Administration of NaHS to cardiomyocytes increased glucose uptake by these cells [174, 175], increased the phosphorylation of several components of the insulin/IGF-1 signal pathway, like insulin receptor, PI3K and Akt [175], and improved glucose metabolism [174, 176]. H2S regulated vaso-relaxation in spontaneously hypertensive rats through the inhibition of FOXO1 and FOXO3 phosphorylation, which resulted in their nuclear translocation and their binding to target gene promotors [177]. H2S was also shown to function as an endogenous regulator of PTEN, the main antagonist of the PI3K-Akt axis in the insulin/IGF-1 signal pathway, by modifying PTEN activity through S-sulfhydration [178, 179]. Following incubation with H2S, oral keratinocyte stem cells increased their PTEN expression [180]. These studies indicate a regulatory role for H2S in the insulin/IGF-1 signaling network.
More Info on Edmund Optics ... Produces optics, imaging, and photonics technology. Supports R&D, electronics, semiconductor, pharmaceutical, biomedical, and ...
MultiPin connectortypes
Electricalpin connectortypes
Happy 20th Anniversary - Stemless Wine Glass Gifts for Women & Men - 20 Year Anniversary Party Decor - Large Glasses $19.95 USD
Genomic stability can be affected by H2S, but the data are conflicting. In Chinese hamster ovaries, human colonic epithelial cells and human lung fibroblasts, H2S may exert genotoxic actions [75, 76]. Human fibroblasts treated with NaHS showed a concentration-dependent increase in the formation of micronuclei, indicating DNA damage, and were propelled towards cell-cycle arrest and apoptotic death through DNA damage responses involving p53, p21 and the apoptosis regulators, Bax and cytochrome c [76]. H2S was genotoxic at concentrations of 0.25 mmol/L, which is similar to the physiological concentration observed in mouse colon (0.2–1 mmol/L [77]) and human feces (0.2–3.4 mmol/L H2S [78]) [75]. However, the genotoxic concentration is much higher than the human plasma concentration of H2S (10–100 μmol/L) [79, 80]. High, genotoxic H2S concentration in the gut, possibly due to production by commensal sulfate-reducing bacteria, may play a role in the genomic instability and the acquisition of mutations observed in colorectal cancer [75]. However, it must be noted that the reliable measurement of H2S is still a topic of discussion, so it is not known whether these mentioned plasma concentrations are accurate or comparable between studies [32, 81–85].
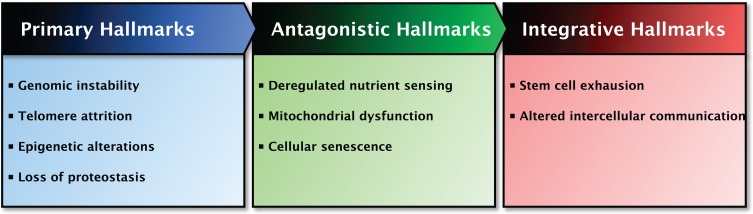
In total, nine hallmarks of aging were proposed which together are thought to determine the aging phenotype (Figure 1) [15]. The criteria for the hallmarks are that each hallmark should be manifested during normal aging and that its experimental intervention should both accelerate or retard the normal aging process, depending on the intervention [15]. However, not all hallmarks currently meet up to all the criteria as amelioration of the aging process is not always successful [15]. The hallmarks are interconnected, making it difficult to determine the relative contribution of each hallmark to aging. There is some degree of hierarchy between the hallmarks of aging and therefore they are divided into three categories: primary, antagonistic and integrative hallmarks [15]. Primary hallmarks, including genomic instability, telomere attrition, epigenetic alterations and loss of proteostasis, are considered to be the primary cause of damage at cellular level which progressively accumulates with time. In response, antagonistic hallmarks that are principally beneficial and mitigate damage may become deleterious themselves, progressively contributing to aging. Deregulated nutrient sensing, mitochondrial dysfunction and cellular senescence belong to this category. Finally, the integrative hallmarks arise when the tissue homeostatic mechanisms cannot compensate for the damage caused by the previous two categories. The integrative hall-marks, stem cell exhaustion and altered intercellular communication, are ultimately responsible for the age-related functional declines [15]. All nine hallmarks of aging and the effects of H2S on each hallmark will be comprehensively discussed in this review.
Research on the aging process of sometimes distantly related model organisms – including yeast, nematodes, flies, mice and humans – led to the idea that lifespan regulation and aging are modulated by common and conserved mechanisms in many, if not all species [3, 5]. The discovery that exogenous hydrogen sulfide (H2S) prolongs the lifespan of the nematode Caenorhabditis elegans [6] has led to new insights in its role in health and disease, suggesting a relationship between H2S and aging [7]. This gaseous molecule was not considered to be physiologically relevant until the 1990s. Since then, increasing evidence showed its beneficial effects in several disease models, including age-related pathologies [8]. The age-dependent decline in plasma H2S levels found in human subjects 50 to 80 years of age supports the link between H2S and aging [9]. However, the precise relationship between H2S and aging is still largely unknown as both the role of H2S in the aging process as well as the effects of aging on the metabolism of H2S are not fully understood yet [8, 10]. This review outlines the current knowledge on the relationship between hydrogen sulfide and aging so as to determine the potential beneficial effects of hydrogen sulfide in aging and age-related pathologies.
Hydrogen sulfide affects the maintenance of cellular protein homeostasis. NaHS treatment suppresses the increased protein synthesis and aggregation in cultured brain slices from Zucker diabetic rats, normalizing proteostasis and counteracting oxidative stress [142]. NaHS inhibited the formation of advanced glycation end products, which disrupt proteostasis, in human neuroblastoma SH-SY5Y cells exposed to D-galactose [143]. Exposure to H2S protected animals against hypoxia-induced disruptions of proteostasis, like protein aggregation and cytotoxicity, and reversed the detrimental effects of hypoxia on protein homeostasis [144]. Cardioprotective effects of heat-shock protein-90 were observed in chemical hypoxia-induced injury to rat H9c2 cardiomyoblasts after NaHS exposure, inhibiting oxidative stress and preserving mitochondrial function [145]. These effects might be a result of improvement to cellular proteostasis. H2S may also affect proteins through S-sulfhydration, which is a form of posttranslational modification, leading to changes in intracellular signaling [146].
The proposed nine hallmarks of aging are categorized based on common characteristics and their contribution to aging. Left panel: The primary hallmarks of aging are the hallmarks regarded as the primary cause of cellular damage. Middle panel: The antagonistic hallmarks of aging are those hallmarks considered to be part of compensatory or antagonistic responses to damage. These hallmarks initially mitigate the damage, but eventually can become deleterious themselves. Right panel: The integrative hallmarks of aging are the end result of the two previously described categories and are ultimately responsible for the functional decline associated with aging. The interactions between the categories are indicated at the top of the panels.
The altered secretome of senescent cells, with enhanced amounts of pro-inflammatory cytokines and chemokines, normally stimulates the innate immune system to eliminate senescent cells but may also contribute to or even accelerate the aging process when the turnover system becomes inefficient [15, 226, 227, 244]. The accumulation of senescent cells was shown in some, but not all, tissues with age demonstrating that this process became inefficient [15, 245]. During cell-cycle arrest, senescence can also be prevented by a process called assisted cycling, in which the cellular disabilities are mended [226, 246].
Several nutrient signaling pathways have been linked to aging. Restricting the dietary caloric intake is the most effective intervention known to slow down aging and extend lifespan in many species [14, 148]. The insulin/IGF1 signaling pathway is the most conserved glucose sensing and the best-characterized aging-controlling pathway [15]. Attenuations at different levels in this pathway were associated with lifespan extension in animals [149–152] and humans [153, 154]. Among the downstream modulators of the insulin/IGF-1 network, the FOXO transcription factors are the most relevant [149], as they modulate the expression of pro-longevity genes in the nucleus [155–157]. Their translocation to the cytosol, due to phosphorylation, inactivates these transcriptional targets [150]. Paradoxically, declines in the insulin/IGF-1 network were shown during both normal and premature aging [158].
Besides the need to prevent functional decline of stem cells, organisms need to prevent the excessive proliferation of stem cells which could result in exhaustion of the stem cell compartment and accelerate aging [15]. Several physiological alterations observed during aging are thought to reflect an attempt of the organism to preserve the quiescence of stem cells [15].
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
The age-associated alterations in intercellular communication also explain the interorgan coordination of aging, in which lifespan-extending manipulations in one tissue were described to retard the aging process in other tissues [279–282]. Manipulation of the gut microbiome was also suggested to retard aging as it may affect the efficiency and function of the host immune system and exert systemic metabolic effects [15, 283, 284].
WirePinConnectors Kit
Others point out the positive effects of H2S on genome stability. Hydrogen sulfide attenuates DNA damage in human endothelial cells and fibroblasts by increasing MEK1 S-sulfhydration, ERK1/2 and PARP-1 activity leading to the activation of DNA damage repair mechanisms and protection from cellular senescence [86]. The CSE/H2S pathway is important in genome stability and cell proliferation, as its inhibition in hepatoma cells decreased their proliferation, enhanced ROS production and mitochondrial disruption, pronounced DNA damage and increased apoptosis [87]. Proliferation was decreased due to downregulation of ERK1/2 activity [87]. The increased apoptosis after H2S signal inhibition was associated with the activation of p53, p21, Bax and other pro-apoptotic factors [87]. Thus, both high [76] as well as low H2S levels [87] can induce apoptosis. Two other studies showed protective effects of H2S containing water [88] and a mitochondrially-targeted H2S donor (AP39) [89] against oxidative stress and oxidative DNA damage in peripheral blood mononuclear cells of Alzheimer's patients and a murine brain microvascular endothelial cell line, respectively, again showing the positive effects of increased intracellular H2S on nuclear and mitochondrial genome stability. These and other studies also described improved cell viability [88, 90] and affected mitochondrial activity [89] after H2S administration.
As research on aging is as old as the hills, it has witnessed the emergence of several new fields of research. Among them is the investigation on gasotransmitters: small, labile and endogenously-generated gaseous transmitters that mediate physiology and disease. Nitric oxide (NO) and carbon monoxide (CO) were the first two identified gasotransmitters and based on their biology several criteria to classify gasotransmitters were proposed [16]. Gasotransmitters diffuse freely across membranes and have well-defined functions in signal transduction, acting on specific cellular and molecular targets at physiologically relevant concentrations. Their endogenous production is regulated by specific substrates and enzymes in mammalian cells and their function can be mimicked by exogenously applied counterparts or obtained from the diet [16].
The enzymatic production of H2S depends on three enzymes, namely cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS) and 3-mercapto-pyruvate sulfurtransferase (3MST) (Figure 2) [35, 36]. The vitamin B6-dependent enzymes CBS and CSE are normally localized in the cytoplasm and translocate to mitochondria under stressful conditions to promote the mitochondrial production of H2S and adenosine triphosphate (ATP) [37–39]. The other H2S producing enzyme 3MST is mainly localized in mitochondria and is found in the cytoplasm to a minor extent [40, 41].
Treatment with H2S or influencing the transsulfuration pathways may become an intervention to prevent, delay or reverse aging and accompanying symptoms and pathologies. However, both beneficial as well as detrimental effects of H2S application were shown in several hallmarks, indicating that administration of H2S dictates great caution. The underlying pathways of both the aging process and the (patho-) physiological processes of H2S signaling need to be studied more extensively. Combining these studies may shed new lights on the role of H2S in aging is needed in order to determine the possible preventive and therapeutic potential of H2S on the process of aging.
Whereas the previously discussed hallmarks of aging mainly focused on age-associated intracellular alterations, the interaction between cells also changes with age. As a result of aging, the extracellular environment of cells may change, altering their intercellular communication [15]. The changed secretome of senescent cells is an example of such an alteration in the paracrine interaction between cells [15, 226, 227, 244]. Senescent cells were also described to induce senescence in neighboring cells via juxtacrine signaling and processes involving ROS [271]. Alterations on several other levels of intercellular communication, like endocrine, neuroendocrine or neuronal signaling [150, 272–274], during aging have been described [15]. The age-associated alterations in the insulin/IGF-1 network are examples of how the neuroendocrine communication can be altered with age. Another important example of altered intercellular communication is ‘inflammaging’, in which mammalian aging is accompanied by a pro-inflammatory phenotype [275]. Some age-related pathologies, like obesity, type 2 diabetes [152] and atherosclerosis [276], were associated with inflammation. Numerous age-associated alterations, such as the pro-inflammatory secretome of senescent cells, the activation of NF-κB signaling and a failing autophagy response during aging, contribute to inflammaging [196, 275]. The function of the adaptive immune system declines with age due to immuno-senescence leading to impaired clearance of infectious agents and infected, damaged or senescent cells, which may, in turn, aggravate the aging phenotype [15, 277]. Sirtuins may also contribute to this phenotype by changing the expression of inflammatory genes [278].
Several studies showed that H2S treatment alters intercellular communication. Hydrogen sulfide was proposed to mediate inflammation, however both pro- and anti-inflammatory actions are described [8]. At low, physiological concentrations, H2S is predominantly anti-inflammatory, whereas high H2S concentrations may promote inflammation [28, 285]. Pro-inflammatory cytokines increase endogenous H2S production in chondrocytes and mesenchymal progenitor cells [286], whereas L-cysteine or NaHS administration decreased NF-κB phosphorylation and reduced the secretion of pro-inflammatory cytokines in human U937 monocytes treated with high-glucose [181]. Exogenous H2S protects H9c2 cardiac cells against high glucose-induced inflammation and injury by inhibiting the NF-κB/IL-1β pathway [287]. Consistently, NaHS decreased the secretion of pro-inflammatory cytokines in mice with severe hepatic ischemia and reperfusion injury, via mechanisms involving Nrf2 [288]. Neuroinflammation and vascular inflammation were modulated by H2S, in part through the activation of AMPK and the reduction of oxidative stress by H2S [181, 183]. Beside the effects of H2S on inflammation, H2S depresses gap junction intercellular communication, which inhibited human platelet aggregation in vitro [289]. Diffusion of H2S from an H2S-producing cell into neighboring cells affects ion management and intracellular processes [8, 146, 290]. H2S regulates various ion channels and transporters by sulfhydrating specific cysteine residues of subunits of these protein complexes [8, 146, 290].
The cell-cycle of damaged cells can be arrested and cells become senescent in order to prevent unrestricted growth [225, 226]. Senescent cells undergo characteristic phenotypic alterations, including activation of tumor-suppressors, epigenetic changes and changes to their secretome [15, 226, 227]. Cellular senescence was suggested to be a beneficial compensatory response to eliminate damaged, potentially oncogenic cells from tissues in order to replace them to re-establish cell numbers [15]. Experimental studies support this principle where a mild enhancement of the senescence pathways extended longevity in mice [228, 229] and elimination of the involved pathways was beneficial for mice with premature aging diseases [70, 230, 231].
Interestingly, the dedifferentiation of several cancer cells, a process in which cancer cells regain characteristics of undifferentiated stem cells, was characterized by the accumulation of H2S and the upregulation of H2S producing enzymes [270]. Reducing the H2S production in these cells reversed their ability to dedifferentiate, whereas the accumulation of H2S induced their dedifferentiation [270]. Altogether, these studies show that stem cell maintenance and H2S signaling are connected.
Despite the fact that all gasotransmitters have their own specific functions and targets, it is plausible that interactions take place between their signaling roles [21]. Indeed, gasotransmitters share several targets and functions with one another [16, 20]. The activities of these shared targets are, however, modulated through different mechanisms [8, 22, 23]. Another possibility is that the eventual outcome of the actions of gasotransmitters is the same despite the fact that different targets and mechanisms were involved [17]. Studies also revealed crosstalk between gaso-transmitters [22–24] in such a way that “each gas may antagonize, reciprocally regulate or potentiate the cellular effects of each other through their production, downstream molecular targets and direct chemical interactions” [21]. Their interconnectedness complicates the search for the specific effects of each individual gasotransmitter, as observed beneficial effects after the modulation of one gasotransmitter might be the result of its crosstalk with other gasotransmitters.
Hydrogen sulfide affects at least one pathway involved in almost all hallmarks of aging. A direct effect of H2S on pathways involved in telomere attrition was not shown, however the effects of H2S on genome stability might also be beneficial for telomere maintenance, by protecting the integrity of the genome. This is indicated by the interrupted line between H2S and telomere attrition. Physiological levels of H2S were shown to prevent the dysregulation of the pathways associated with aging.
Several calcium and potassium channels associated with biological processes, such as cardiac contraction, sensory transduction, inflammation and neuro-protection, were shown to be manipulated by H2S [8, 146, 290]. These effects of H2S have also been observed in the cerebral microcirculation in response to hypoxia, where H2S produced in astrocytes causes vasodilatation by diffusion into the contractile pericytes that surround the arterioles [291]. Impaired H2S production might thus result in vascular dysfunction and other pathophysiologies [8, 146]. Taken together, these studies demonstrate that H2S may have anti-aging properties by altering intercellular communication.
Mitochondria are the major producers of ROS, as its synthesis is an inevitable by-product of oxidative phosphorylation. The mitochondrial function declines with age, leading to increased electron leakage and ROS production and reduced ATP generation [196], which may, in turn, cause extra damage to the mitochondrial genome and further decline mitochondrial function. This vicious cycle, known as the mitochondrial free radical theory of aging [201], may lead to cellular energy depletion and ultimately to cell death [202, 203]. Since its proposal, numerous studies were performed to test the theory, generating inconsistent and conflicting results [204]. This led to a reconsideration of the role of ROS in aging [205]. In contrast to what was seen in severe mitochondrial stress, low physiological levels of intracellular ROS, maintained within a narrow range [206], have signaling functions in many cellular and systemic physiological processes [198, 207], inducing beneficial long-lasting metabolic and biochemical changes that may actually improve the cellular fitness [15, 197, 208, 209]. The phenomenon in which the exposure to low levels of a stressor induces compensatory biological processes, whereas higher levels disrupt homeostasis, is known as hormesis [209, 210]. Thus, dependent on its intracellular level, ROS can have both pathogenic, aging accelerating as well as lifespan increasing actions [208, 211, 212].




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500