Dome Interior Light Type - Free Shipping on Orders Over ... - custom dome lights
The telescope designer in Herschel grasped the significance immediately: If light and radiant heat have the same optical properties, if they exhibit the same behavior in their interactions with matter, might that indicate they are the same quantity? He notes: “
The heating was greatest in red, but the curve did not appear to reach a maximum in the visible spectrum. Instead, the readings seemed to point somewhere in the dark region beyond red. He felt compelled to follow this trend.
Something about the temperature readings clearly bothered him. He had expected, as he found, that the readings would be different for the various colors. But the measurements also showed something he did not expect: a trend, rather than a peak.
He discovered that radiant heat has the same optical properties as light. He confirmed his hypothesis that heating power is not equally distributed across the spectrum. He performed the first radiometric measurement of spectral radiant power across the visible into the infrared and found it to be a smooth, continuous curve.
Plumbing pipe lighting hardware for you lighting construction project. To make good connection in a safe and compliant manner we can supply a range of parts ...
This observation is remarkable: Yellow-green is near the wavelength where the Sun’s radiant power is a maximum and is exactly where the eye’s sensitivity is greatest.
Feeling he had proven that both radiant heat and light are not equally distributed across the colors, and with measurement results showing their differences, Herschel should have been ready to move on to applying these results to his problem of viewing the Sun. But he didn’t do so immediately. Instead, he returned to the temperature data.
20241122 — Selecting a light source from a vast range of types, sizes, and colors is challenging even for the most experienced machine vision practitioner.
A computer atmospheric model was used to calculate the distribution of solar irradiance that illuminated Herschel’s prism, and the curve was normalized for comparison. Herschel was meticulous in recording his temperatures, but although he owned a number of prisms of both crown and flint glass with a variety of angles, he did not record what was used in his first experiments. The curve shown assumes his prism was of crown, which was common in 1800, and had a 60-degree angle.
By the following year, he was grinding his own mirrors to build larger and better quality telescopes, and spending his nights studying the heavens. Herschel’s craftsmanship rapidly took his hobby to public recognition as the foremost telescope manufacturer of his time. His fame peaked after he discovered the planet Uranus in 1781, which led to his appointment as the king’s astronomer.
Light spectrum
If an instrument responds nonuniformly, as most do, then the response shape is impressed on the received radiation in a way that cannot be extricated without independent knowledge of how the radiation is distributed and how the instrument responds. Lacking this concept, Herschel assumed the spectra he measured accurately represented how the radiation was distributed.
When speaking about microscopes, there are two types of microscope still in use, such as optical microscope, also known as light microscope.
The illumination experiment also went well. He did 10 separate experiments with objects viewed in different colors. He attempted to distinguish between the color having the maximum of illumination and that having the sharpest resolution or “distinctness.” He was unable to reach a conclusion about resolution, but for illumination, he was able to state:
Figure 3. The electromagnetic spectrum stretches from gamma rays to radio waves, but human beings can directly sense only two small segments. Our eyes see light, a narrow band of wavelengths centered approximately where the Sun’s radiant power is at its maximum. Our skin feels warmth across the spectrum, but mainly from infrared, which spans the range of wavelengths between light and microwaves. Everyday experience alone would not lead us to believe they are the same quantity.
Most encyclopedias and physics books credit the great British astronomer Sir William Herschel with the discovery of infrared radiation in 1800. It’s a good story, but it is not strictly correct—it trivializes the true significance of what Herschel found.
These differences may be why the connection was not made for so many centuries in spite of much experimentation. Perhaps this counterintuitive association is why the connection was found almost by accident by a person with no formal scientific training.
To evaluate Herschel’s spectra, we need to see how the Sun appeared from the village of Slough at the time of his measurements. Herschel did not record the date and time, but analysis of the curve shape indicates the data likely came from his first experiments, and thus were probably taken sometime in late February or early March. Slough lies at latitude 51.5 degrees north, which would make the solar zenith around 61 degrees (29 degrees above the horizon) at local noon.
Herschel embarked on an extensive instrument-building program to examine and measure each property. To the original prism and mercury thermometers he added a variety of lenses and mirrors in a dozen different configurations and an extensive array of transparent materials to compare transmission.
From these data, Herschel felt that he had proven his hypothesis about heating being unequally distributed and could move on to the illumination experiment. As he stated in his initial conclusion:
Electromagneticwaves
By describing the rays in terms of momentum, Herschel was not anticipating the discoveries of quantum physics, still a century in the future. Photons at infrared wavelengths do have less energy than those in the visible band. As a result, they do “have such a momentum as to be unfit for vision.” He was not looking a century ahead but a century back—to Isaac Newton’s experiments with light in the late 1600s.
Following his appointment, which came with an annual salary of £200, Herschel was able to devote all his time to astronomy. He and his sister, Caroline, settled in the town of Slough, near Windsor Castle. A condition of his appointment was that he be available to King George III and the royal family any time they wished to view the stars.
The boundary between light and infrared is determined by the long-wavelength limit of the human eye’s response. Everyday experience would not lead us to believe light and infrared are the same kind of energy. Indeed, two compelling pieces of evidence suggest, logically, that they are not related.
Lightwave
In a paper read before the Royal Society on March 27, 1800, Herschel called this warmth felt at a distance “radiant heat.” This description is still a good working term for infrared radiation. The term “infrared” did not enter scientific vocabulary until the 1880s. Infra is Latin for “below,” but researchers have been unable to trace the source of who initially coined the name.
To find the maximum of illumination, he directed colors onto a variety of small objects that he viewed through a 27-power microscope. From the brightness and clarity of what he saw, he judged the relative illumination.
If the maximum lay outside the visible spectrum, then the heating was not from light but from something else. Herschel used the expression “invisible light,” cautiously phrasing it in a way that indicated he knew it to be an oxymoron. (If rays are invisible, then they aren’t light.) As he expressed it:
The 'heat' they look for is infrared light, which is emitted by objects depending on their temperature (and surface characteristics). Mirrors ...
He placed one thermometer in the light and kept the other two in darkness to measure the room’s ambient temperature. Herschel understood there were, as he expressed it, “causes acting in different ways” (in other words, conduction and convection) that affected the stabilization temperature of the thermometers, and he wanted to quantify the heating caused by the light alone.
The vast electromagnetic spectrum stretches from gamma rays (whose wavelengths can be smaller than the width of an atom) to radio waves (whose wavelengths can reach thousands of kilometers). Of this range, humans are only able to directly sense radiation in two small bands. Our eyes see light, which occupies a narrow sliver of wavelengths from 0.4 to 0.7 micrometers, centered approximately where the Sun’s radiant power is at its maximum. Our skin feels warmth mainly from infrared, which spans the range of wavelengths between light and microwaves, up to about 1,000 micrometers.
If Herschel’s measurements had been made in summer, when the Sun is higher in the sky, the maximum he found would have been closer to the center of the visible spectrum. But in winter, with a longer atmospheric path, the maximum of the spectral irradiance (the incident power density as a function of wavelength) is pushed toward red due to atmospheric scattering of the shorter wavelengths.
He took readings with his thermometer, following the upward trend to a maximum and beyond, until the heating began to diminish. The tone of Herschel’s second paper is one of excitement and confidence in his findings. Throughout his experiments, he ventures few opinions that are not firmly supported by data, but he concludes this paper with an argument based on philosophy:
He measured the effects of scattering, and found, correctly, that light scatters more than infrared. Herschel attributed the difference to light and heat having different natures, rather than as evidence of similar behavior in a different interaction with matter (as scattering is dependent on wavelength).
Figure 10. The output of any sensor is the product of two curves: the spectral distribution of received radiation and the spectral response of the sensor. The narrowness of the eye’s response (black dashed line) produces a product curve (red line) that is nearly the same shape as the response curve. The result is more a map of how the human eye responds than of how light is distributed. Herschel’s error was assuming that this curve should resemble his temperature measurement.
This argument would not be credible today and probably sounded weak even in 1800, but it was a way of bringing his quest to a close. Herschel was surely disappointed, but he had achieved more than he or any contemporary realized.
With his device set up on a sunny day, Herschel methodically took temperature measurements, first comparing the thermometers’ readings at ambient conditions to ensure baseline agreement. It was cold in the room. His starting temperatures averaged 43.5 degrees Fahrenheit. After some experimentation, he settled on using his own thermometer with its half-inch-diameter bulb exposed to the light and used the larger of the borrowed ones as the ambient reference.
In spite of its displacement, the shape of Herschel’s curve confirms his first hypothesis that the heating power of sunlight is not equally distributed across the spectrum. The fact that his curve is continuous in its transition into the infrared supports his second hypothesis that light and radiant heat are the same quantity.
Figure 5. Herschel directed the dispersed colors from a prism onto a piece of cardboard with a slit that allowed only a single color to pass. To measure relative heating, he kept one thermometer in the light and the other in shadow.
By November 1800, Herschel was approaching the end of what was possible to achieve given the knowledge and technology of his time. He was also almost certainly feeling pressure to return to astronomy.
The title of this paper has an intriguing echo of Newton. In Newton’s Opticks (1730), his Proposition II, Theorem II is titled “The Light of the Sun consists of Rays differently Refrangible.”
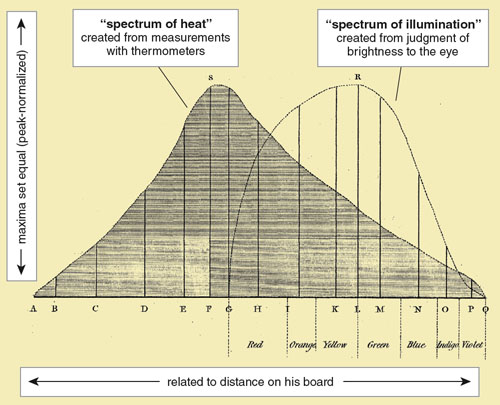
The presentation of data can strongly influence their interpretation. Even today, it is difficult to look at Herschel’s graph without the impression that light and radiant heat are two different types of rays. Herschel’s curves are both accurate, but they are of different, almost unrelated quantities and should not be graphed together. His error was not in his basic data but in his assumption that the curves were comparable.
Any spectrum created from what the eye sees will be zero outside the eye’s limits. Inside its limits, the power received at any wavelength will be weighted by the eye’s sensitivity to that color. The greatest sensitivity, as Herschel correctly determined, is yellow-green at a wavelength of 0.555 micrometers.
Human color vision is more complicated than shown, but the International Commission on Illumination curve for the light-adapted (or photopic) eye illustrates the concept (see figure 10). The sensation of sight is so rich in information that we don’t often think about how narrow is the slice of the electromagnetic spectrum that we see. Because of its narrowness, the shape of the response curve and that of its product with the solar spectrum are nearly identical.
This observation led to the thought that different colors might, in Herschel’s words, “have the power of heating bodies very unequally distributed among them.” Herschel further reasoned that if the heating power were unequally distributed, the illuminating power might be as well. There might be a single best color for seeing, and it might be different than the one for maximum heating. Knowing these qualities would help him find the best filter to view the Sun.
If Leslie’s differential thermometer (he called it a “photometer”) found no heating beyond the visible as he claimed, then it was badly in error. This point was later proven dramatically by independent experiments conducted by the Royal Society. To deflect criticism, Herschel switched from the term “radiant heat” to “the rays that occasion heat.”
USA Lighting Brand to the World We ship from Texas, USA. 1710 Texas Ave, El Paso, Texas 79901. eqlight.com. Follow. Message ...
Figure 4. Herschel’s mental leap to connect light and radiant heat had to overcome everyday experience with each energy: If light and heat are the same, we’d expect to find them together, and sometimes radiant heat can be found alone (as with hot coffee or the human body, at middle and bottom insets). A century after Herschel, quantum theory explained that radiant power had a wave-shaped distribution whose position on the spectrum shifts upward and toward shorter wavelengths with increasing temperature (blue arrow). Around 700 degrees Celsius, the shortwavelength edge of the curve pushes sufficiently into the visible range for the human eye to see a red glow, such as from an electric grill (top inset). Infrared images were taken in the band of wavelengths indicated by the gray box.
We all discover infrared at a young age when we feel warmth at a distance from a hot object, and we know that these rays are invisible—warmth can be felt in total darkness. What Herschel discovered was subtler than the existence of invisible radiation. He found the first solid evidence that light and infrared are the same quantity that we know today to be electromagnetic radiation. Through a series of simple experiments, Herschel found the first piece in one of the great puzzles of physics that took another century to solve.
Today, the nonuniform response of a spectrometer can be corrected. But such calibration requires knowledge of wavelength and a source of known radiant power, neither of which were concepts in Herschel’s time. The displacement of his heat curve is not erroneous in itself; it was actually serendipitous because it created the temperature trend that led him into the infrared.
Electromagneticspectrumwavelength
Light was also a contentious issue. Herschel’s brash assertions about radiant heat and light had stepped on the toes of conventional belief. With light, he again adopted a cautious stance, but this time he countered with a challenge calculated to silence his critics:
It was a question that dominated Herschel’s thoughts and effort for much of the rest of the year. He must have worked rapidly, because just 9 days after writing his first paper and 10 days before he formally presented it, he wrote a second, shorter paper to the Royal Society titled “Experiments on the Refrangibility of the Invisible Rays of the Sun.”
Company: Smart Vision Lights, LLC ; Location: 5113 Robert Hunter Dr, Norton Shores, Michigan, 49441-6547, US ; Company Phone: (231) 722-1199 ; Industry: Commercial ...
Visiblelight spectrum
Refraction is the change in direction of a ray as it enters or exits a transparent medium that causes a change in velocity, such as between air and glass. Dispersion is the effect of refraction on multiple wavelengths, causing different rays to refract at differing angles. We see the effects of the dispersion of light most commonly from rainbows and prisms. Herschel didn’t think of light in terms of wavelength, but as a lens-maker, he was very familiar with the effects of dispersion and how to correct for it to produce lenses that minimize what is known today as chromatic aberration, where different colors converge to a focus at varying distances from the lens. As he wrote:
Black light refers to ultraviolet or infrared radiation that is invisible to the eye. Black light fixtures emit long wavelength UV-A ultraviolet light and are ...
Herschel’s graph was the product of imagination, insight and months of painstaking work, but it was fatally misleading. Its appearance was probably the deciding factor in his conclusion that light and radiant heat are fundamentally different after all. As he wrote (with the area “ASQA” encompassing the spectrum of heat and “GRQG” the spectrum of illumination):
Herschel was missing a concept, unknown in 1800, but fundamental to radiometry today: the effect of sensor response. The reading obtained with any instrument or sensor, including the human eye, results from the product of two curves: the distribution of the received power and the curve of instrument response, which includes the spectral transmission of all optical elements.
Figure 1. An image of infrared radiation from a hot mug is colored with red, orange and yellow shades to show heat, but hot objects on their own often do not show any visible signs of their emissions. Although it is now common knowledge that both infrared and visible light are part of the electromagnetic spectrum, connecting the two seemingly disparate energy sources took some intricate experiments—and unfettered curiosity—by the famous 18th-century British astronomer Sir William Herschel.
As a result, Herschel’s spectrum of illumination is more a map of how the human eye responds to colors than how light is actually distributed. He saw the shape of his curve as evidence that light is not equally distributed across the spectrum. His hypothesis was correct, but his data did not show it. He would have gotten an almost identical curve even if the distribution were uniform.
Herschel did not have a scientist’s insight into the causes of these phenomena, and he had limited ability to formulate mathematical descriptions of his findings. His strengths were his practical knowledge of optics combined with craftsmanship in the fabrication of instruments and his powers of observation.

Herschel’s reputation as an astronomer probably helped ensure that his papers were favorably received by most scientists, but not by all. His third paper opens on a decidedly defensive note. He appears to have been attacked by a person he refers to as a “celebrated author,” who took offense at the phrase “radiant heat.” This detractor may have been John Leslie, who was considered an authority on heat and clearly resented the intrusion of an amateur into his domain. In a letter published in A Journal of Natural Philosophy, Chemistry, and the Arts by William Nicholson, Leslie wrote:
First, we experience light and infrared differently with different senses. We see light, perceiving different wavelengths as different colors, but we feel infrared only as warmth. Second, light and infrared aren’t always found together. Most sources of light also emit infrared, but infrared is often found by itself. A familiar example is an electric grill not hot enough to glow: If the room is completely dark, we can still feel the warmth from the grill, but we can’t see it.
The paper’s final proposition asks whether radiant heat, if sufficiently strong, is able to stimulate vision. This question is critical because the complete answer explains why light and infrared are usually together but infrared can be present without light. With his 18th experiment, Herschel determined beyond doubt that increasing its power cannot make infrared visible.
Spectrometers today have much higher resolution, greater sensitivity and faster response, but the basic functional elements are the same as Herschel’s. Prisms are still used, but better resolution is usually obtained through wave interference —wavelengths are separated by constructive and destructive interference, where their waves either add together or cancel out. The detector today would be a cryogenically cooled semiconductor—much smaller, faster and more sensitive than a mercury thermometer. But Herschel’s instrument, in the hands of the careful experimenter that he was, gave surprisingly accurate measurements.
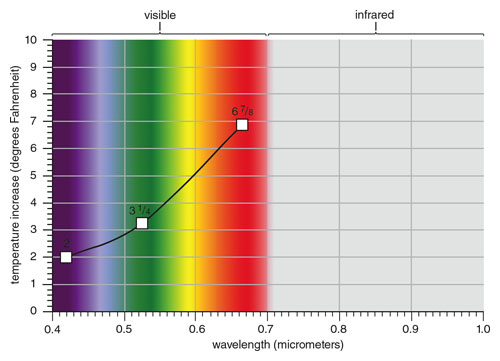
Herschel positioned the measuring thermometer in the band of colored light for each reading. At each color position, he allowed the thermometer to stabilize for 10 minutes before taking a reading. He took a series of measurements, starting with red, which gave an average reading of 67/8 degrees above ambient. Green gave 31/4, and violet light gave a 2-degree average increase.
In the end, the misleading appearance of his graph and the different sensations of seeing light and feeling heat won out over all of his carefully collected evidence, leading him to conclude that the two energies are not the same after all. Actually, he never reached a firm conclusion. The closest Herschel came was to again invoke philosophy, this time to argue against his original thought:
Our present understanding of electromagnetic radiation grew from Herschel’s simple measurements of temperature in sunlight to the unification of the spectrum mathematically by James Clerk Maxwell in 1861 and, ultimately, to Max Planck’s formulation of the quantum theory in 1900. Herschel could not conclusively prove that light and radiant heat are the same quantity, but his experiments provided very strong evidence and were the first piece of the puzzle that others later built on.
Herschel was not originally a scientist. He rose from obscurity as a German immigrant in a military band to become an accomplished musician and composer. In 1773, at age 34, six years after moving to Bath to take a position teaching music and playing in concerts, he did something that changed his life and fortunes. He bought a small telescope and a book on astronomy.
It wasn’t the concept of invisible rays that so interested Herschel. What captivated him were the properties of these rays. It was clear to him that radiant heat had the same optical properties of “refrangibility” (“refraction” in modern usage) and dispersion as light.
Electromagneticspectrum
Herschel accepted without question Newton’s beliefs on the “corpuscular” nature of light. A strong case for light being a wave had been made by Newton’s contemporary, Christiaan Huygens, but the theory of light as streams of minute particles dominated science at the time, especially in Britain. This viewpoint changed within the next 15 years, but at that moment, Herschel thought of light as particles that had more or less “efficacy” in their effect on matter.
Herschel’s foray from astronomy to infrared was a fortuitous tangent from his effort to find the best color for a filter that would allow him to safely view the Sun. His speculations and conclusions were often contradictory: Many were wrong, but some were extraordinarily prescient. The story of Herschel’s experiments is that of the role of human perception in scientific discovery, as well as the conflict between conventional beliefs and concepts never before encountered.
Thorlabs' High-Intensity Fiber-Coupled Light Sources are designed for illumination of brightfield microscopy setups and general-purpose laboratory use. A ...
His final paper, presented November 6, 1800, contained the first graph made showing the spectral distributions of visible light and infrared radiation. He called these curves the “spectrum of illumination” and the “spectrum of heat.” On the vertical axis, Herschel plotted measured temperature and perceived brightness. He set their maxima equal (a format called peak-normalization) to compare the relative extent and shape of the distributions and the location of their maxima. For the horizontal axis, not having the concept of wavelength, he used distance in relation to where the visible colors fell. The horizontal axis is reversed from today’s convention of increasing wavelength from left to right.
In more than 200 experiments, he recorded page after page of readings using every available illuminating source viewed through different combinations of mirrors, prisms and lenses. He confirmed again and again, in every way he could test, that light and radiant heat have the same optical properties.
For the spectrum of illumination, it must have seemed logical to Herschel to plot this on the same graph because his original objective was to find a filter that would maximize light while minimizing heat. His curve was accurate, but it did not show what he thought.
Figure 7. Herschel had to modify his instrument to follow the heating trend into the invisible, as this drawing from his second paper shows. Lacking the concept of wavelength, his only reference was in relation to where the last visible light fell. He marked off five parallel lines and positioned the board so the edge of red fell at the first line. His temperature readings increased to a maximum at approximately half an inch beyond red and diminished beyond. The experiment demonstrated that radiant heat has the same optical properties as light.
The frantic pace of his later experiments may have caused him to miss or misinterpret connections, especially after he began to look for differences instead of similarities. He did a detailed experiment showing that the focal length of a refractive converging lens was longer for heat than for light, without realizing that the difference in focal length is caused by the same dispersion as a prism.
Figure 6. With his first temperature readings, Herschel believed he had proven the heating power of light was not equally distributed across the visible spectrum, as he had found the greatest heating from red. But his readings also showed a trend that appeared to point toward a maximum somewhere in the dark region beyond red. It was a trend that he felt compelled to follow.
Herschel had observed features on the Sun’s surface for a number of years, presenting a paper on the Sun and fixed stars to the Royal Society in 1794. Being able to observe sunspots with a large telescope without damaging his eyes had long been a challenge. Through experiments with different combinations of colored and darkened glass, Herschel observed, as he noted in this paper:
Comparing his spectrum of heat with the solar spectrum shows that Herschel made remarkably good measurements considering the limitations of his instrument and that he had no idea how a solar spectrum should look. His curve is displaced toward longer wavelengths because of the dispersion curve of glass.
Figure 9. Comparing Herschel’s “spectrum of heat” with that of sunlight shows his measurement was surprisingly accurate. His curve is displaced toward longer wavelengths due to the dispersion curve of glass. Without the concept of wavelength and a known radiation source, this effect could not have been corrected in Herschel’s time. Herschel’s curve confirms his first hypothesis that the heating power of sunlight is not equally distributed across the spectrum. And, the fact that his curve is continuous in its transition across the visible and into the infrared strongly supports his second hypothesis that light and radiant heat are the same quantity.
identify whichwaves of theelectromagneticspectrumreach earth.
To discuss our articles or comment on them, please share them and tag American Scientist on social media platforms. Here are links to our profiles on Twitter, Facebook, and LinkedIn.
The best information about Herschel’s experiments is found in his original papers. His recorded data and many of his comments appear to have been taken directly from his lab notes, and their freshness and authenticity come through even today. The biggest challenge reading his work is to follow his line of reasoning through many digressions and pages of raw temperature data.
Newton clearly had great influence on Herschel. The latter’s approach was nearly identical to Newton’s experiments using a prism placed in a window to project colors onto a wall. Herschel took Newton’s basic qualitative method of viewing the spectrum and turned it into a quantitative instrument. He may have felt—justifiably—that he was continuing the work Newton had begun with the colors of light by extending the concept of “different refrangibility” to the rays of the Sun that lay beyond the visible colors.
Herschel began his second experiment by slightly modifying his spectrometer to take temperature readings into the dark area on his board, beyond red. His only reference was in relation to where the colors fell on the table. He marked off five parallel lines spaced half an inch apart on a sheet of white paper, with the first line at the edge of the band of red light. Thus anchored to the edge of the visible, Herschel ventured into the darkness beyond.
Attollo Engineering has partnered with LightPath Technologies Inc. (NASDAQ: LPTH) to design and supply freeform optical components for Attollo's LiDAR products.
His first instrument consisted of three components: a prism set in a south- facing window to catch the sunlight and direct and disperse the colors down onto a table; a small panel of cardboard with a slit wide enough for only a single color to pass through; and three mercury- in-glass thermometers (of which he used two) with their bulbs blackened to better absorb light. Thermometers were not common household items in 1800, but Herschel had one of his own and borrowed two more from a colleague.
Herschel’s third paper proposed seven comparisons between light and radiant heat. The first concerns two human senses. The next five are interactions with matter that were known in 1800: reflection, refraction, “different refrangibility” (dispersion), transmission through “diaphanous bodies” (transparent media) and scattering from rough surfaces.
How manywavesare intheelectromagneticspectrum
BOA Quick Setup · How to Set Up the BOA Smart Camera Vision System · Webinar: "Selecting the Right Camera for Vision Applications".
Figure 8. Herschel’s final paper in 1800 contained the first graph showing the spectral distributions of visible light and radiant heat (what he called the “spectrum of illumination” and the “spectrum of heat”). Its two curves are of different, almost unrelated quantities and their misleading appearance on the same graph ultimately led Herschel to wrongly conclude that rays of light and radiant heat are of a different nature after all.
The electromagnetic spectrum is simply the full range of wave frequencies that characterizes solar radiation. Although we are talking about light, most of the electromagnetic spectrum cannot be detected by the human eye. Even satellite detectors only capture a small portion of the entire electromagnetic spectrum. From longest to shortest wavelengths, the spectrum is usually divided into the following sections: radio, microwave, infrared, visible, ultraviolet, x-ray, and gamma-ray radiation. Humans can only see a narrow band of visible light, which is a small fraction of the electromagnetic spectrum. We perceive this radiation as the colors of the rainbow ranging from red to violet, with reds having longer wavelengths (~ 0.7 micrometers) and violet having shorter wavelengths (~ 0.4 micrometers). But keep in mind how “long” these wavelengths really are. One micrometer (μm) is equal to one-millionth of a meter which is approximately 1/100 the diameter of a human hair – that’s small! For comparison, microwaves are on the order of one centimeter long and television and radio waves have lengths greater than one meter.
Relating the index of refraction to wavelength, the dispersion curve causes refraction to spread the wavelengths across the target board nonuniformly. At each position, moving toward longer wavelengths, the spectral width or band of wavelengths that the thermometer receives becomes progressively wider. A wider spectral width contains more power, which increases the reading. The result gives readings at longer wavelengths that are weighted more heavily than those at shorter wavelengths.
Drawing on his experience making telescopes, Herschel built an instrument to test his hypothesis. He made what we would call a spectrometer, or more precisely, a spectroradiometer: an instrument to measure the magnitude of radiant power at different wavelengths.
The criticism he received did not slow his experiments, but these attacks may have had an impact. By the second part of his final paper, his emphasis changed from finding evidence supporting the similarity between light and radiant heat to that supporting their difference.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500