Brightfield Illumination of Large Field Sizes - bright field illumination
Ultra Violet Light Replacement Bulb and Sleeve for all UltraPure Systems. Includes: (1) UV Replacement Bulb (1) UV Replacement Quartz Sleeve.
Hinkleylighting
Except where otherwise noted, content on this site is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
... lights, two ring lights, one ring light, etc. I don't ... A tungsten balanced (or variable colour) softbox/led panel for your fill light.
Tgllighting
Choose from a great selection of LED spotlights and downlights for bathrooms, kitchens or bedrooms (GU10, MR16, fire rated lights).
N Mojarad · 2015 · 110 — In the case of extreme ultraviolet (EUV) lithography at 13.5 nm wavelength, this limit is set by light diffraction and is ≈3.5 nm. In the semiconductor industry ...
Rplighting
Aqua Ultraviolet uses the latest technology for disinfecting water with a modern design. Shop our online store for UV light filters for swimming pools.
The John A. Dutton Institute for Teaching and Learning Excellence is the learning design unit of the College of Earth and Mineral Sciences at The Pennsylvania State University.
EliteLighting
This courseware module is offered as part of the Repository of Open and Affordable Materials at Penn State. Except where otherwise noted, content on this site is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The College of Earth and Mineral Sciences is committed to making its websites accessible to all users, and welcomes comments or suggestions on access improvements. Please send comments or suggestions on accessibility to the site editor. The site editor may also be contacted with questions or comments about this Open Educational Resource.
Atoms and molecules can absorb radiation (a photon) only if their structure has an energy difference between levels that matches the photon’s energy (hc/λ). Otherwise, the atom or molecule will not absorb the light. Once the molecule has absorbed the photon, it can either lose a photon and go back to its original lower energy level; or it can break apart if the photon energy is greater than the chemical bond holding the molecule together; or it can collide with other molecules, such as N2 or O2, and transfer energy to them while it goes back to its lower energy level. Collisions happen often, so the energy of the absorbed photon is often transferred to thermal energy.
The College of Earth and Mineral Sciences is committed to making its websites accessible to all users, and welcomes comments or suggestions on access improvements. Please send comments or suggestions on accessibility to the site editor. The site editor may also be contacted with questions or comments about this Open Educational Resource.
The PRO300 is a targeted red light therapy device that delivers targeted 660nm and 850nm wavelengths for various benefits including skin rejuvenation, wound ...
Americanlighting
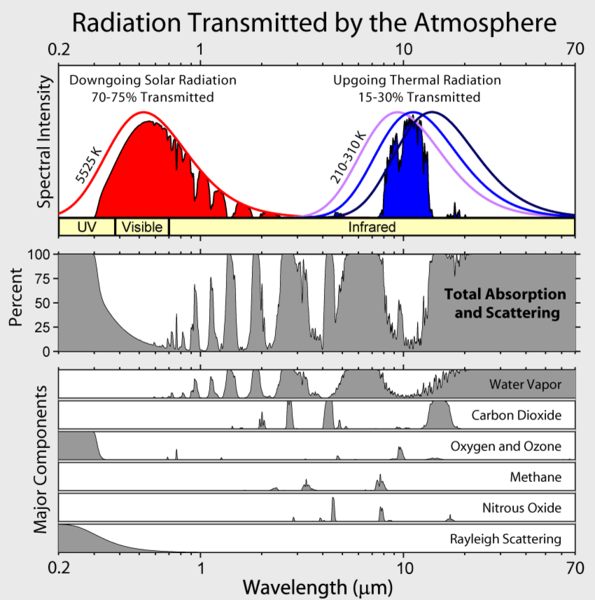
Note that Earth's outgoing infrared irradiance is limited to a few atmospheric "windows" and the irradiance at all other wavelengths is strongly absorbed, mostly by water vapor, but also by carbon dioxide, ozone, nitrous oxide, methane, and other more trace gases that aren't shown in the figure above.
The atmosphere absorbs a significant amount of radiation in the infrared but rather little in the visible. Also, we see that gases absorb strongly at some wavelengths and not at others. Why is this?
Light Vision ... Light Vision was a bi-monthly Australian photography magazine that existed between 1977 and 1978.
KuzcoLighting
Capitallighting
We offer over 200 standard sizes of LED machine vision lights with prices published for transparency. Customized lights are also available at standard light ...
Kichlerlighting
The following bulleted list is a crash course in absorption by the electrons in atoms and molecules. Refer to the figure below the box.
To answer this question, we need to look at the configurations of the electrons that are zooming around atoms and molecules. More than 100 years ago, scientists began using prisms to disperse the light from the sun and from flames containing different elements. While the sun gave the colors of the rainbow, the flames had light in very distinct lines or bands. This puzzle was finally resolved a little more than 100 years ago with the invention of quantum mechanics, which basically says that the electrons zooming around atoms and molecules and the vibrations and rotations of molecules can have only discrete energies that are governed by rules of conservation of angular momentum.
Diffusing light means to soften it by reducing glare and harsh shadows. In diffused lighting, subjects will appear to have shadows with very soft edges or no ...

The absorption cross section, σ, varies significantly over the width of the absorption line. So it is possible for all the radiation to be absorbed in the middle of the line but very little absorbed in the “wings.”
The Clear Films block 99% of all UV below 400nm, while allowing more than 80% visible light transmission above 400nm.




 Ms.Cici
Ms.Cici 
 8618319014500
8618319014500